Differences between bacterial communities in the gut of a soil-feeding termite (Cubitermes niokoloensis) and its mounds
- PMID: 17574999
- PMCID: PMC1950997
- DOI: 10.1128/AEM.02616-06
Differences between bacterial communities in the gut of a soil-feeding termite (Cubitermes niokoloensis) and its mounds
Abstract
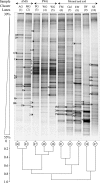
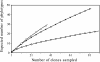
In tropical ecosystems, termite mound soils constitute an important soil compartment covering around 10% of African soils. Previous studies have shown (S. Fall, S. Nazaret, J. L. Chotte, and A. Brauman, Microb. Ecol. 28:191-199, 2004) that the bacterial genetic structure of the mounds of soil-feeding termites (Cubitermes niokoloensis) is different from that of their surrounding soil. The aim of this study was to characterize the specificity of bacterial communities within mounds with respect to the digestive and soil origins of the mound. We have compared the bacterial community structures of a termite mound, termite gut sections, and surrounding soil using PCR-denaturing gradient gel electrophoresis (DGGE) analysis and cloning and sequencing of PCR-amplified 16S rRNA gene fragments. DGGE analysis revealed a drastic difference between the genetic structures of the bacterial communities of the termite gut and the mound. Analysis of 266 clones, including 54 from excised bands, revealed a high level of diversity in each biota investigated. The soil-feeding termite mound was dominated by the Actinobacteria phylum, whereas the Firmicutes and Proteobacteria phyla dominate the gut sections of termites and the surrounding soil, respectively. Phylogenetic analyses revealed a distinct clustering of Actinobacteria phylotypes between the mound and the surrounding soil. The Actinobacteria clones of the termite mound were diverse, distributed among 10 distinct families, and like those in the termite gut environment lightly dominated by the Nocardioidaceae family. Our findings confirmed that the soil-feeding termite mound (C. niokoloensis) represents a specific bacterial habitat in the tropics.
Figures
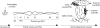


References
-
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. - PubMed
-
- Beare, M. H., D. C. Coleman, D. A. Crossley, Jr., P. F. Hendrix, and E. P. Odum. 1995. A hierarchical approach to evaluating the significance of soil biodiversity to biogeochemical cycling. Plant Soil 170:5-22.
-
- Bignell, D. E., J. M. Anderson, and R. Crosse. 1991. Isolation of facultatively aerobic actinomycetes from the gut, parent soil and mound materials of the termites Procubitermes aburiensis and Cubitermes severus. FEMS Microb. Ecol. 85:151-160.
-
- Bignell, D. E., and P. Eggleton. 2000. Termites in ecosystems, p. 363-387. In T. Abe, D. E. Bignell, and M. Higashi (ed.), Termites: evolution, sociality, symbioses, ecology, vol. 1. Kluwer Academic, Dordrecht, The Netherlands.
-
- Brauman, A. 2000. Effect of gut transit and mound deposit on soil organic matter transformations in the soil feeding termite: a review. Eur. J. Soil Biol. 36:117-125.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

