Positive feedback between Dia1, LARG, and RhoA regulates cell morphology and invasion
- PMID: 17575049
- PMCID: PMC1891425
- DOI: 10.1101/gad.424807
Positive feedback between Dia1, LARG, and RhoA regulates cell morphology and invasion
Abstract
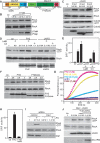
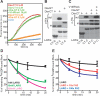
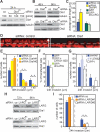
The RhoA-effector Dia1 controls actin-dependent processes such as cytokinesis, SRF transcriptional activity, and cell motility. Dia1 polymerizes actin through its formin homology (FH) 2 domain. Here we show that Dia1 acts upstream of RhoA independently of its effects on actin assembly. Dia1 binds to the leukemia-associated Rho-GEF (LARG) through RhoA-dependent release of Dia1 autoinhibition. The FH2 domain stimulates the guanine nucleotide exchange activity of LARG in vitro. Our results reveal that Dia1 is necessary for LPA-stimulated Rho/ROCK signaling and bleb-associated cancer cell invasion. Thus, Dia1-dependent RhoA activation constitutes a positive feedback mechanism to modulate cell behavior.
Figures


References
-
- Brandt D.T., Marion S., Griffiths G., Watanabe T., Kaibuchi K., Grosse R., Marion S., Griffiths G., Watanabe T., Kaibuchi K., Grosse R., Griffiths G., Watanabe T., Kaibuchi K., Grosse R., Watanabe T., Kaibuchi K., Grosse R., Kaibuchi K., Grosse R., Grosse R. Dia1 and IQGAP1 interact in cell migration and phagocytic cup formation. J. Cell Biol. 2007 (in press) - PMC - PubMed
-
- Chikumi H., Barac A., Behbahani B., Gao Y., Teramoto H., Zheng Y., Gutkind J.S., Barac A., Behbahani B., Gao Y., Teramoto H., Zheng Y., Gutkind J.S., Behbahani B., Gao Y., Teramoto H., Zheng Y., Gutkind J.S., Gao Y., Teramoto H., Zheng Y., Gutkind J.S., Teramoto H., Zheng Y., Gutkind J.S., Zheng Y., Gutkind J.S., Gutkind J.S. Homo- and hetero-oligomerization of PDZ-RhoGEF, LARG and p115RhoGEF by their C-terminal region regulates their in vivo Rho GEF activity and transforming potential. Oncogene. 2004;23:233–240. - PubMed
-
- Faix J., Grosse R., Grosse R. Staying in shape with formins. Dev. Cell. 2006;10:693–706. - PubMed
-
- Gomez T.S., Kumar K., Medeiros R.B., Shimizu Y., Leibson P.J., Billadeau D.D., Kumar K., Medeiros R.B., Shimizu Y., Leibson P.J., Billadeau D.D., Medeiros R.B., Shimizu Y., Leibson P.J., Billadeau D.D., Shimizu Y., Leibson P.J., Billadeau D.D., Leibson P.J., Billadeau D.D., Billadeau D.D. Formins regulate the actin-related protein 2/3 complex-independent polarization of the centrosome to the immunological synapse. Immunity. 2007;26:177–190. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
