Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization
- PMID: 17575055
- PMCID: PMC1891431
- DOI: 10.1101/gad.436307
Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization
Abstract
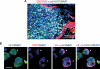
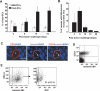
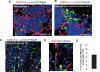
Tumors build vessels by cooption of pre-existing vasculature and de novo recruitment of bone marrow (BM)-derived endothelial progenitor cells (EPCs). However, the contribution and the functional role of EPCs in tumor neoangiogenesis are controversial. Therefore, by using genetically marked BM progenitor cells, we demonstrate the precise spatial and temporal contribution of EPCs to the neovascularization of three transplanted and one spontaneous breast tumor in vivo using high-resolution microscopy and flow cytometry. We show that early tumors recruit BM-derived EPCs that differentiate into mature BM-derived endothelial cells (ECs) and luminally incorporate into a subset of sprouting tumor neovessels. Notably, in later tumors, these BM-derived vessels are diluted with non-BM-derived vessels from the periphery, which accounts for purported differences in previously published reports. Furthermore, we show that specific ablation of BM-derived EPCs with alpha-particle-emitting anti-VE-cadherin antibody markedly impaired tumor growth associated with reduced vascularization. Our results demonstrate that BM-derived EPCs are critical components of the earliest phases of tumor neoangiogenesis.
Figures




References
-
- Aozuka Y., Koizumi K., Saitoh Y., Ueda Y., Sakurai H., Saiki I., Koizumi K., Saitoh Y., Ueda Y., Sakurai H., Saiki I., Saitoh Y., Ueda Y., Sakurai H., Saiki I., Ueda Y., Sakurai H., Saiki I., Sakurai H., Saiki I., Saiki I. Anti-tumor angiogenesis effect of aminopeptidase inhibitor bestatin against B16–BL6 melanoma cells orthotopically implanted into syngeneic mice. Cancer Lett. 2004;216:35–42. - PubMed
-
- Asahara T., Murohara T., Sullivan A., Silver M., van der Zee R., Li T., Witzenbichler B., Schatteman G., Isner J.M., Murohara T., Sullivan A., Silver M., van der Zee R., Li T., Witzenbichler B., Schatteman G., Isner J.M., Sullivan A., Silver M., van der Zee R., Li T., Witzenbichler B., Schatteman G., Isner J.M., Silver M., van der Zee R., Li T., Witzenbichler B., Schatteman G., Isner J.M., van der Zee R., Li T., Witzenbichler B., Schatteman G., Isner J.M., Li T., Witzenbichler B., Schatteman G., Isner J.M., Witzenbichler B., Schatteman G., Isner J.M., Schatteman G., Isner J.M., Isner J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. - PubMed
-
- Asahara T., Takahashi T., Masuda H., Kalka C., Chen D., Iwaguro H., Inai Y., Silver M., Isner J.M., Takahashi T., Masuda H., Kalka C., Chen D., Iwaguro H., Inai Y., Silver M., Isner J.M., Masuda H., Kalka C., Chen D., Iwaguro H., Inai Y., Silver M., Isner J.M., Kalka C., Chen D., Iwaguro H., Inai Y., Silver M., Isner J.M., Chen D., Iwaguro H., Inai Y., Silver M., Isner J.M., Iwaguro H., Inai Y., Silver M., Isner J.M., Inai Y., Silver M., Isner J.M., Silver M., Isner J.M., Isner J.M. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. - PMC - PubMed
-
- Baumann C.I., Bailey A.S., Li W., Ferkowicz M.J., Yoder M.C., Fleming W.H., Bailey A.S., Li W., Ferkowicz M.J., Yoder M.C., Fleming W.H., Li W., Ferkowicz M.J., Yoder M.C., Fleming W.H., Ferkowicz M.J., Yoder M.C., Fleming W.H., Yoder M.C., Fleming W.H., Fleming W.H. PECAM-1 is expressed on hematopoietic stem cells throughout ontogeny and identifies a population of erythroid progenitors. Blood. 2004;104:1010–1016. - PubMed
-
- Bertolini F., Shaked Y., Mancuso P., Kerbel R.S., Shaked Y., Mancuso P., Kerbel R.S., Mancuso P., Kerbel R.S., Kerbel R.S. The multifaceted circulating endothelial cell in cancer: Towards marker and target identification. Nat. Rev. Cancer. 2006;6:835–845. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
