Gravin regulates mesodermal cell behavior changes required for axis elongation during zebrafish gastrulation
- PMID: 17575056
- PMCID: PMC1891432
- DOI: 10.1101/gad.1535007
Gravin regulates mesodermal cell behavior changes required for axis elongation during zebrafish gastrulation
Abstract
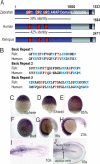
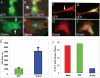
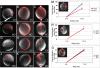
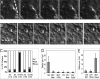
Convergent extension of the mesoderm is the major driving force of vertebrate gastrulation. During this process, mesodermal cells move toward the future dorsal side of the embryo, then radically change behavior as they initiate extension of the body axis. How cells make this transition in behavior is unknown. We have identified the scaffolding protein and tumor suppressor Gravin as a key regulator of this process in zebrafish embryos. We show that Gravin is required for the conversion of mesodermal cells from a highly migratory behavior to the medio-laterally intercalative behavior required for body axis extension. In the absence of Gravin, paraxial mesodermal cells fail to shut down the protrusive activity mediated by the Rho/ROCK/Myosin II pathway, resulting in embryos with severe extension defects. We propose that Gravin functions as an essential scaffold for regulatory proteins that suppress the migratory behavior of the mesoderm during gastrulation, and suggest that this function also explains how Gravin inhibits invasive behaviors in metastatic cells.
Figures



References
-
- Bakkers J., Kramer C., Pothof J., Quaedvlieg N.E., Spaink H.P., Hammerschmidt M., Kramer C., Pothof J., Quaedvlieg N.E., Spaink H.P., Hammerschmidt M., Pothof J., Quaedvlieg N.E., Spaink H.P., Hammerschmidt M., Quaedvlieg N.E., Spaink H.P., Hammerschmidt M., Spaink H.P., Hammerschmidt M., Hammerschmidt M. Has2 is required upstream of Rac1 to govern dorsal migration of lateral cells during zebrafish gastrulation. Development. 2004;131:525–537. - PubMed
-
- Blaser H., Reichman-Fried M., Castanon I., Dumstrei K., Marlow F.L., Kawakami K., Solnica-Krezel L., Heisenberg C.P., Raz E., Reichman-Fried M., Castanon I., Dumstrei K., Marlow F.L., Kawakami K., Solnica-Krezel L., Heisenberg C.P., Raz E., Castanon I., Dumstrei K., Marlow F.L., Kawakami K., Solnica-Krezel L., Heisenberg C.P., Raz E., Dumstrei K., Marlow F.L., Kawakami K., Solnica-Krezel L., Heisenberg C.P., Raz E., Marlow F.L., Kawakami K., Solnica-Krezel L., Heisenberg C.P., Raz E., Kawakami K., Solnica-Krezel L., Heisenberg C.P., Raz E., Solnica-Krezel L., Heisenberg C.P., Raz E., Heisenberg C.P., Raz E., Raz E. Migration of zebrafish primordial germ cells: A role for Myosin contraction and cytoplasmic flow. Dev. Cell. 2006;11:613–627. - PubMed
-
- Carreira-Barbosa F., Concha M.L., Takeuchi M., Ueno N., Wilson S.W., Tada M., Concha M.L., Takeuchi M., Ueno N., Wilson S.W., Tada M., Takeuchi M., Ueno N., Wilson S.W., Tada M., Ueno N., Wilson S.W., Tada M., Wilson S.W., Tada M., Tada M. Prickle 1 regulates cell movements during gastrulation and neuronal migration in zebrafish. Development. 2003;130:4037–4046. - PubMed
-
- Charras G.T., Yarrow J.C., Horton M.A., Mahadevan L., Mitchison T.J., Yarrow J.C., Horton M.A., Mahadevan L., Mitchison T.J., Horton M.A., Mahadevan L., Mitchison T.J., Mahadevan L., Mitchison T.J., Mitchison T.J. Non-equilibration of hydrostatic pressure in blebbing cells. Nature. 2005;435:365–369. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
