Direct transition metal-catalyzed functionalization of heteroaromatic compounds
- PMID: 17576484
- PMCID: PMC3674790
- DOI: 10.1039/b606984n
Direct transition metal-catalyzed functionalization of heteroaromatic compounds
Abstract
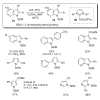
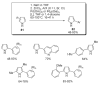
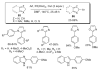
During the last two decades there has been considerable growth in the development of catalytic reactions capable of activating unreactive C-H bonds. These methods allow for the synthesis of complex molecules from easily available and cheaper precursors in a fewer number of steps. Naturally, the development of C-H activation methods for direct functionalization of heterocyclic molecules, invaluable building blocks for pharmaceutical and synthetic chemistry and material science, has received substantial attention as well. This critical review summarizes the progress made in this field until November 2006 (117 references).
Figures

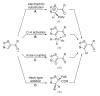
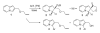
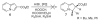
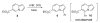
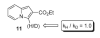
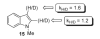

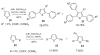
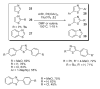
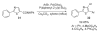

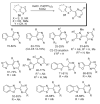
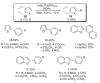








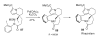
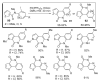
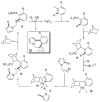

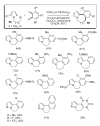

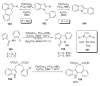



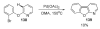

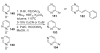
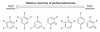
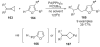
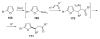
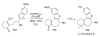


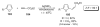

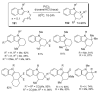
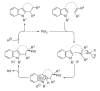
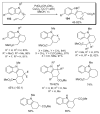
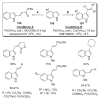

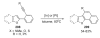
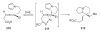





References
-
-
For reviews on C–H activation, see: Godula K, Sames D. Science. 2006;312:67. Jia CG, Kitamura T, Fujiwara Y. Acc. Chem. Res. 2001;34:844. Ritleng V, Sirlin C, Pfeffer M. Chem. Rev. 2002;102:1731. Kakiuchi F, Murai S. Acc. Chem. Res. 2002;35:826.
-
-
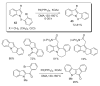
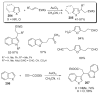
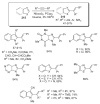
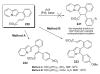
- Ames DE, Bull D. Tetrahedron. 1982;38:383.
-
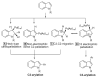
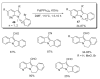
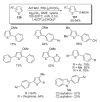
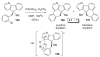
- Pivsa-Art S, Satoh T, Kawamura Y, Miura M, Nomura M. Bull. Chem. Soc. Jpn. 1998;71:467.
-
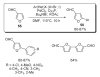
- Okazawa T, Satoh T, Miura M, Nomura M. J. Am. Chem. Soc. 2002;124:5286. - PubMed
-
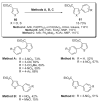
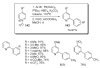
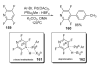
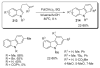
- Glover B, Harvey KA, Liu B, Sharp MJ, Tymoschenko M. Org. Lett. 2003;5:301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

