Fine-tuning of intrinsic N-Oct-3 POU domain allostery by regulatory DNA targets
- PMID: 17576670
- PMCID: PMC1935007
- DOI: 10.1093/nar/gkm453
Fine-tuning of intrinsic N-Oct-3 POU domain allostery by regulatory DNA targets
Abstract
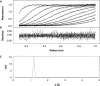
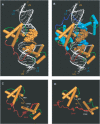
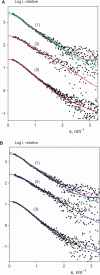
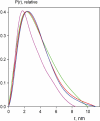
The 'POU' (acronym of Pit-1, Oct-1, Unc-86) family of transcription factors share a common DNA-binding domain of approximately 160 residues, comprising so-called 'POUs' and 'POUh' sub-domains connected by a flexible linker. The importance of POU proteins as developmental regulators and tumor-promoting agents is due to linker flexibility, which allows them to adapt to a considerable variety of DNA targets. However, because of this flexibility, it has not been possible to determine the Oct-1/Pit-1 linker structure in crystallographic POU/DNA complexes. We have previously shown that the neuronal POU protein N-Oct-3 linker contains a structured region. Here, we have used a combination of hydrodynamic methods, DNA footprinting experiments, molecular modeling and small angle X-ray scattering to (i) structurally interpret the N-Oct-3-binding site within the HLA DRalpha gene promoter and deduce from this a novel POU domain allosteric conformation and (ii) analyze the molecular mechanisms involved in conformational transitions. We conclude that there might exist a continuum running from free to 'pre-bound' N-Oct-3 POU conformations and that regulatory DNA regions likely select pre-existing conformers, in addition to molding the appropriate DBD structure. Finally, we suggest that a specific pair of glycine residues in the linker might act as a major conformational switch.
Figures




Similar articles
-
Differential effects of phosphorylation on DNA binding properties of N Oct-3 are dictated by protein/DNA complex structures.J Mol Biol. 2007 Jul 20;370(4):687-700. doi: 10.1016/j.jmb.2007.04.072. Epub 2007 May 10. J Mol Biol. 2007. PMID: 17543985
-
Linker length and composition influence the flexibility of Oct-1 DNA binding.EMBO J. 1997 Apr 15;16(8):2043-53. doi: 10.1093/emboj/16.8.2043. EMBO J. 1997. PMID: 9155030 Free PMC article.
-
POU domain factors in neural development.Adv Exp Med Biol. 1998;449:39-53. doi: 10.1007/978-1-4615-4871-3_4. Adv Exp Med Biol. 1998. PMID: 10026784 Review.
-
Protein interaction surface of the POU transcription factor UNC-86 selectively used in touch neurons.EMBO J. 2000 Jul 17;19(14):3694-703. doi: 10.1093/emboj/19.14.3694. EMBO J. 2000. PMID: 10899123 Free PMC article.
-
The virtuoso of versatility: POU proteins that flex to fit.J Mol Biol. 2000 Oct 6;302(5):1023-39. doi: 10.1006/jmbi.2000.4107. J Mol Biol. 2000. PMID: 11183772 Review.
References
-
- Chua G, Robinson MD, Morris Q, Hughes TR. Transcriptional networks: reverse-engineering gene regulation on a global scale. Curr. Opin. Microbiol. 2004;7:638–646. - PubMed
-
- Gunewardena S, Zhang Z. Accounting for structural properties and nucleotide co-variations in the quantitative prediction of binding affinities of protein-DNA interactions. Pac. Symp. Biocomput. 2006:379–390. - PubMed
-
- Garvie CW, Wolberger C. Recognition of specific DNA sequences. Mol. Cell. 2001;8:937–946. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

