Quantitative analysis of an anaphase B switch: predicted role for a microtubule catastrophe gradient
- PMID: 17576796
- PMCID: PMC2064360
- DOI: 10.1083/jcb.200611113
Quantitative analysis of an anaphase B switch: predicted role for a microtubule catastrophe gradient
Abstract
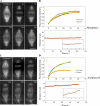
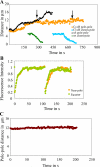
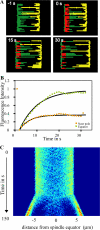
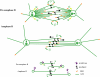
Anaphase B in Drosophila embryos is initiated by the inhibition of microtubule (MT) depolymerization at spindle poles, which allows outwardly sliding interpolar (ip) MTs to drive pole-pole separation. Using fluorescence recovery after photobleaching, we observed that MTs throughout the preanaphase B spindle are very dynamic and display complete recovery of fluorescence, but during anaphase B, MTs proximal to the poles stabilize and therefore display lower recovery than those elsewhere. Fluorescence microscopy of the MT tip tracker EB1 revealed that growing MT plus ends localize throughout the preanaphase B spindle but concentrate in the overlap region of interpolar MTs (ipMTs) at anaphase B onset. None of these changes occurred in the presence of nondegradable cyclin B. Modeling suggests that they depend on the establishment of a spatial gradient of MT plus-end catastrophe frequencies, decreasing toward the equator. The resulting redistribution of ipMT plus ends to the overlap zone, together with the suppression of minus-end depolymerization at the poles, could constitute a mechanical switch that initiates spindle elongation.
Figures



References
-
- Ashburner, M. 1989. Drosophila: A Laboratory Handbook. Cold Spring Harbor Laboratory Press. Cold Spring Harbor, NY. 1331 pp.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

