Essential and distinct roles for cdc42 and rac1 in the regulation of Schwann cell biology during peripheral nervous system development
- PMID: 17576798
- PMCID: PMC2064365
- DOI: 10.1083/jcb.200610108
Essential and distinct roles for cdc42 and rac1 in the regulation of Schwann cell biology during peripheral nervous system development
Abstract
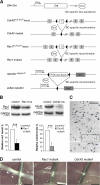
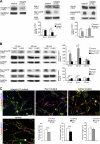
During peripheral nervous system (PNS) myelination, Schwann cells must interpret extracellular cues to sense their environment and regulate their intrinsic developmental program accordingly. The pathways and mechanisms involved in this process are only partially understood. We use tissue-specific conditional gene targeting to show that members of the Rho GTPases, cdc42 and rac1, have different and essential roles in axon sorting by Schwann cells. Our results indicate that although cdc42 is required for normal Schwann cell proliferation, rac1 regulates Schwann cell process extension and stabilization, allowing efficient radial sorting of axon bundles.
Figures





Comment in
-
Myelination: all about Rac 'n' roll.J Cell Biol. 2007 Jun 18;177(6):953-5. doi: 10.1083/jcb.200705105. J Cell Biol. 2007. PMID: 17576794 Free PMC article. Review.
References
-
- Atanasoski, S., L. Notterpek, H.Y. Lee, F. Castagner, P. Young, M.U. Ehrengruber, D. Meijer, L. Sommer, E. Stavnezer, C. Colmenares, and U. Suter. 2004. The protooncogene Ski controls Schwann cell proliferation and myelination. Neuron. 43:499–511. - PubMed
-
- Benninger, Y., H. Colognato, T. Thurnherr, R.J. Franklin, D.P. Leone, S. Atanasoski, K.A. Nave, C. ffrench-Constant, U. Suter, and J.B. Relvas. 2006. Beta1-integrin signaling mediates premyelinating oligodendrocyte survival but is not required for CNS myelination and remyelination. J. Neurosci. 26:7665–7673. - PMC - PubMed
-
- Bradley, W.G., and M. Jenkison. 1975. Neural abnormalities in the dystrophic mouse. J. Neurol. Sci. 25:249–255. - PubMed
-
- Buttery, P.C., and C. ffrench-Constant. 1999. Laminin-2/integrin interactions enhance myelin membrane formation by oligodendrocytes. Mol. Cell. Neurosci. 14:199–212. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

