Fas (CD95) induces macrophage proinflammatory chemokine production via a MyD88-dependent, caspase-independent pathway
- PMID: 17576821
- PMCID: PMC4492281
- DOI: 10.1189/jlb.1006652
Fas (CD95) induces macrophage proinflammatory chemokine production via a MyD88-dependent, caspase-independent pathway
Abstract
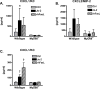
Activation of the prototypical death receptor, Fas (CD95), can induce both caspase-dependent cell death and production of proinflammatory chemokines, leading to neutrophil recruitment and end-organ injury. The precise mechanism(s) by which Fas up-regulates chemokine production and release, is currently unclear. We hypothesized that Fas-induced chemokine release by macrophages is dependent on the MyD88 adaptor molecule and independent of caspase activity. To test this hypothesis, we measured chemokine response to Fas activation both in RAW 264.7 cells with RNAi-attenuated MyD88 expression and in MyD88-deficient primary macrophages. We found that Fas-induced chemokine release was abrogated in the absence of MyD88. In vivo, MyD88(-/-) mice had impaired CXCL1/KC release and polymorphonuclear cell recruitment in response to intratracheal treatment with the Fas-activating monoclonal antibody, Jo-2. Furthermore, Fas-induced chemokine release was not dependent on either IL-1 receptor signaling or on caspase activity. We conclude that MyD88 plays an integral role in Fas-induced macrophage-mediated inflammation.
Figures

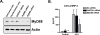
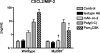



References
-
- Fine A, Anderson NL, Rothstein TL, Williams MC, Gochuico BR. Fas expression in pulmonary alveolar type II cells. Am J Physiol. 1997;273:L64–71. - PubMed
-
- Curtin JF, Cotter TG. Live and let die: regulatory mechanisms in Fas-mediated apoptosis. Cell Signal. 2003;15:983–92. - PubMed
-
- Matute-Bello G, Lee JS, Liles WC, Frevert CW, Mongovin S, Wong V, Ballman K, Sutlief S, Martin TR. Fas-mediated acute lung injury requires fas expression on nonmyeloid cells of the lung. J Immunol. 2005;175:4069–75. - PubMed
-
- Matute-Bello G, Liles WC, Frevert CW, Nakamura M, Ballman K, Vathanaprida C, Kiener PA, Martin TR. Recombinant human Fas ligand induces alveolar epithelial cell apoptosis and lung injury in rabbits. Am J Physiol Lung Cell Mol Physiol. 2001;281:L328–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

