Pharmacokinetics of aztreonam in healthy subjects and patients with cystic fibrosis and evaluation of dose-exposure relationships using monte carlo simulation
- PMID: 17576827
- PMCID: PMC2043218
- DOI: 10.1128/AAC.01522-06
Pharmacokinetics of aztreonam in healthy subjects and patients with cystic fibrosis and evaluation of dose-exposure relationships using monte carlo simulation
Abstract
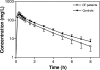
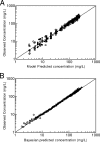
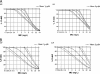
Aztreonam (AZM) is a monobactam antibiotic with a high level of activity against gram-negative micro-organisms, including Pseudomonas aeruginosa. We evaluated AZM pharmacokinetics and pharmacokinetic-pharmacodynamic relationships in patients with cystic fibrosis (CF) and healthy subjects. Pharmacokinetic data in eight CF patients and healthy subjects that were matched for age, gender, weight, and height were obtained and analyzed by using the nonparametric adaptive grid algorithm. Probabilities of target attainment using percentages of time of unbound concentration above the MIC (fT>MIC) were obtained by using a Monte Carlo simulation. AZM total body clearance was significantly higher in CF patients (100.1 +/- 17.1 versus 76.2 +/- 7.4 ml/min in healthy subjects; P < 0.01). The pharmacokinetic parameter estimates for terminal half-life (1.54 +/- 0.17 h [mean +/- the standard deviation]) and volume of distribution (0.20 +/- 0.02 liters/kg in patients with CF patients were not different from those in healthy subjects. Monte Carlo simulations with a target of a fT>MIC of 50 to 60% at a dose of 1,000 mg every 8 h indicated a clinical breakpoint of 4 mg/liter and 1 to 2 mg/liter for healthy subjects and CF patients, respectively. This study using matched controls showed that AZM total body clearance and not the volume of distribution is higher in CF patients as a result of increased renal clearance. Pharmacokinetic parameter estimates in healthy subjects resulted in a clinical susceptibility breakpoint of < or =4 mg/liter for a dose of 1,000 mg every 8 h. Patients suspected of having high clearance rates, such as CF patients, should be monitored closely, with dosing regimens adjusted accordingly.
Figures
References
-
- Ambrose, P. G. 2006. Monte Carlo simulation in the evaluation of susceptibility breakpoints: predicting the future: insights from the society of infectious diseases pharmacists. Pharmacotherapy 26:129-134. - PubMed
-
- Ambrose, P. G., and D. M. Grasela. 2000. The use of Monte Carlo simulation to examine pharmacodynamic variance of drugs: fluoroquinolone pharmacodynamics against Streptococcus pneumoniae. Diagn. Microbiol. Infect. Dis. 38:151-157. - PubMed
-
- Anderson, B. J., K. Allegaert, and N. H. Holford. 2006. Population clinical pharmacology of children: modeling covariate effects. Eur. J. Pediatr. 165:819-829. - PubMed
-
- Bayer, A. S., D. Crowell, C. C. Nast, D. C. Norman, and R. L. Borrelli. 1990. Intravegetation antimicrobial distribution in aortic endocarditis analyzed by computer-generated model. Implications for treatment. Chest 97:611-617. - PubMed
-
- Bonate, P. L. 2001. A brief introduction to Monte Carlo simulation. Clin. Pharmacokinet. 40:15-22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

