Prognostic factors for survival in adult patients with recurrent glioma enrolled onto the new approaches to brain tumor therapy CNS consortium phase I and II clinical trials
- PMID: 17577040
- PMCID: PMC4118746
- DOI: 10.1200/JCO.2006.08.1661
Prognostic factors for survival in adult patients with recurrent glioma enrolled onto the new approaches to brain tumor therapy CNS consortium phase I and II clinical trials
Abstract
Purpose: Prognostic factor analyses have proven useful in predicting outcome in patients with newly diagnosed malignant glioma. Similar analyses in patients with recurrent glioma could affect the design and conduct of clinical trials substantially.
Patients and methods: Between 1995 and 2002, 333 adults with recurrent gliomas were enrolled onto 10 phase I or II trials of systemic or local therapy. The studies had similar inclusion criteria and were conducted within the New Approaches to Brain Tumor Therapy CNS Consortium. Ninety-three percent of the patients have died. Cox proportional hazards (PH) regression and recursive partitioning analysis (RPA) were performed to identify prognostic factors.
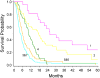
Results: Factors associated with an increased risk of death were increased age, lower Karnofsky performance score (KPS), initial and on-study histologies of glioblastoma multiforme (GBM), corticosteroid use, shorter time from original diagnosis to recurrence, and tumor outside frontal lobe. The final PH model included initial histology of GBM (relative risk [RR] = 2.01), 10-year increase in age (RR = 1.23), KPS less than 80 (RR = 1.54), and corticosteroid use (RR = 1.49). RPA resulted in seven classes. Median survival time was poorest in non-GBM patients with KPS less than 80 or GBM patients, age 50 years, corticosteroid use (4.4 months; 95% CI, 3.6 to 5.4 months); median survival was best in patients with initial histology other than GBM with KPS 80 and tumor confined to the frontal lobe (25.7 months; 95% CI, 18.7 to 52.5), and was 7.0 months (95% CI, 6.2 to 8.0 months) for all patients.
Conclusion: Initial histology, age, KPS, and corticosteroid use are prognostic for survival in recurrent glioma patients. To allow comparisons across phase II trials, enrollment criteria may need to be restricted.
Figures


Comment in
-
Toward a better dialogue between neuro-oncologists and phase I investigators.J Clin Oncol. 2012 Feb 10;30(5):562-3; author reply 563-4. doi: 10.1200/JCO.2011.39.8347. Epub 2012 Jan 3. J Clin Oncol. 2012. PMID: 22215743 No abstract available.
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Huncharek M, Muscat J. Treatment of recurrent high grade astrocytoma; results of a systematic review of 1,415 patients. Anticancer Res. 1998;18:1303–1311. - PubMed
-
- Brem H, Piantadosi S, Burger PC, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas the polymer-brain tumor treatment group. Lancet. 1995;345:1008–1012. - PubMed
-
- Shaw E, Arusell R, Scheithauer B, et al. Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: Initial report of a north central cancer treatment Group/Radiation therapy oncology Group/Eastern cooperative oncology group study. J Clin Oncol. 2002;20:2267–2276. - PubMed
-
- Grossman SA, Fisher JD, Piantadosi S, et al. The New Approaches to Brain Tumor Therapy (NABTT) CNS Consortium: Organization, objectives, and activities. Cancer Control. 1998;5:107–114. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

