Thioredoxin glutathione reductase from Schistosoma mansoni: an essential parasite enzyme and a key drug target
- PMID: 17579510
- PMCID: PMC1892040
- DOI: 10.1371/journal.pmed.0040206
Thioredoxin glutathione reductase from Schistosoma mansoni: an essential parasite enzyme and a key drug target
Erratum in
- PLoS Med. 2007 Aug;4(8):e264
Abstract
Background: Schistosomiasis--infection with helminth parasites in the genus Schistosoma, including S. mansoni--is a widespread, devastating tropical disease affecting more than 200 million people. No vaccine is available, and praziquantel, the only drug extensively utilized, is currently administered more than 100 million people yearly. Because praziquantel resistance may develop it is essential to identify novel drug targets. Our goal was to investigate the potential of a unique, selenium-containing parasite enzyme thioredoxin glutathione reductase (TGR) as a drug target.
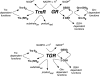
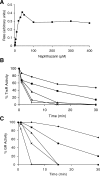
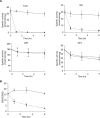
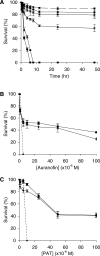
Methods and findings: Using RNA interference we found that TGR is essential for parasite survival; after silencing of TGR expression, in vitro parasites died within 4 d. We also found that auranofin is an efficient inhibitor of pure TGR (Ki = 10 nM), able to kill parasites rapidly in culture at physiological concentrations (5 microM), and able to partially cure infected mice (worm burden reductions of ~60%). Furthermore, two previously used antischistosomal compounds inhibited TGR activity, suggesting that TGR is a key target during therapy with those compounds.
Conclusions: Collectively, our results indicate that parasite TGR meets all the major criteria to be a key target for antischistosomal chemotherapy. To our knowledge this is the first validation of a Schistosoma drug target using a convergence of both genetic and biochemical approaches.
Conflict of interest statement
Figures




References
-
- King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: A meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–1569. - PubMed
-
- Hotez PJ, Molyneux DH, Fenwick A, Ottesen E, Ehrlich Sachs S, et al. Incorporating a rapid-impact package for neglected tropical diseases with programs for HIV/AIDS, tuberculosis, and malaria: A comprehensive pro-poor health policy and strategy for the developing world. PLoS Med. 2006;3:e102. doi: 10.1371/journal.pmed.0030102. - DOI - PMC - PubMed
-
- Fenwick A, Savioli L, Engels D, Bergquist RN, Todd MH. Drugs for the control of parasitic diseases: Current status and development in schistosomiasis. Trends Parasitol. 2003;19:509–515. - PubMed
-
- Cioli D, Pica-Mattoccia L, Archer S. Drug resistance in schistosomes. Parasitol Today. 1993;9:162–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

