Randomised phase III trial of carboplatin plus etoposide vs split doses of cisplatin plus etoposide in elderly or poor-risk patients with extensive disease small-cell lung cancer: JCOG 9702
- PMID: 17579629
- PMCID: PMC2360311
- DOI: 10.1038/sj.bjc.6603810
Randomised phase III trial of carboplatin plus etoposide vs split doses of cisplatin plus etoposide in elderly or poor-risk patients with extensive disease small-cell lung cancer: JCOG 9702
Abstract
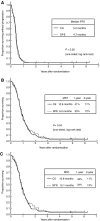
We compared the efficacy and the safety of a carboplatin plus etoposide regimen (CE) vs split doses of cisplatin plus etoposide (SPE) in elderly or poor-risk patients with extensive disease small-cell lung cancer (ED-SCLC). Eligibility criteria included: untreated ED-SCLC; age >/=70 and performance status 0-2, or age <70 and PS 3. The CE arm received carboplatin area under the curve of five intravenously (IV) on day 1 and etoposide 80 mg m(-2) IV on days 1-3. The SPE arm received cisplatin 25 mg m(-2) IV on days 1-3 and etoposide 80 mg m(-2) IV on days 1-3. Both regimens were given with granulocyte colony-stimulating factor support in a 21-28 day cycle for four courses. A total of 220 patients were randomised. Median age was 74 years and 74% had a PS of 0 or 1. Major grade 3-4 toxicities were (%CE/%SPE): leucopenia 54/51, neutropenia 95/90, thrombocytopenia 56/16, infection 7/6. There was no significant difference (CE/SPE) in the response rate (73/73%) and overall survival (median 10.6/9.9 mo; P=0.54). Palliation scores were very similar between the arms. Although the SPE regimen is still considered to be the standard treatment in elderly or poor-risk patients with ED-SCLC, the CE regimen can be an alternative for this population considering the risk-benefit balance.
Figures
References
-
- Ardizzoni A, Favaretto A, Boni L, Baldini E, Castiglioni F, Antonelli P, Pari F, Tibaldi C, Altieri AM, Barbera S, Cacciani G, Raimondi M, Tixi L, Stefani M, Monfardini S, Antilli A, Rosso R, Paccagnella A (2005) Platinum-etoposide chemotherapy in elderly patients with small-cell lung cancer: results of a randomized multicenter phase II study assessing attenuated-dose or full-dose with lenograstim prophylaxis-A Forza Operativa Nazionale Italiana Carcinoma Polmonare and Gruppo Studio Tumori Polmonari Veneto (FONICAP-GSTPV) study. J Clin Oncol 23: 569–575 - PubMed
-
- Carney DN (1995) Carboplatin/etoposide combination chemotherapy in the treatment of poor prognosis patients with small cell lung cancer. Lung Cancer 12(Suppl 3): S77–S83 - PubMed
-
- DeMets DL, Lan KK (1994) Interim analysis: the alpha spending function approach. Stat Med 13: 1341–1352 - PubMed
-
- Evans WK, Radwi A, Tomiak E, Logan DM, Martins H, Stewart DJ, Goss G, Maroun JA, Dahrouge S (1995) Oral etoposide and carboplatin: effective therapy for elderly patients with small cell lung cancer. Am J Clin Oncol 18: 149–155 - PubMed
-
- Findlay MP, Griffin AM, Raghavan D, McDonald KE, Coates AS, Duval PJ, Gianoutsos P (1991) Retrospective review of chemotherapy for small cell lung cancer in the elderly: dose the end justify the means? Eur J Cancer 27: 1597–1601 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical