Erythropoietin blockade inhibits the induction of tumor angiogenesis and progression
- PMID: 17579721
- PMCID: PMC1891087
- DOI: 10.1371/journal.pone.0000549
Erythropoietin blockade inhibits the induction of tumor angiogenesis and progression
Abstract
Background: The induction of tumor angiogenesis, a pathologic process critical for tumor progression, is mediated by multiple regulatory factors released by tumor and host cells. We investigated the role of the hematopoietic cytokine erythropoietin as an angiogenic factor that modulates tumor progression.
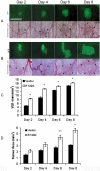
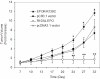
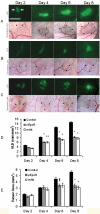
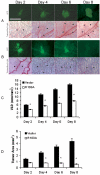
Methodology/principal findings: Fluorescently-labeled rodent mammary carcinoma cells were injected into dorsal skin-fold window chambers in mice, an angiogenesis model that allows direct, non-invasive, serial visualization and real-time assessment of tumor cells and neovascularization simultaneously using intravital microscopy and computerized image analysis during the initial stages of tumorigenesis. Erythropoietin or its antagonist proteins were co-injected with tumor cells into window chambers. In vivo growth of cells engineered to stably express a constitutively active erythropoietin receptor EPOR-R129C or the erythropoietin antagonist R103A-EPO were analyzed in window chambers and in the mammary fat pads of athymic nude mice. Co-injection of erythropoietin with tumor cells or expression of EPOR-R129C in tumor cells significantly stimulated tumor neovascularization and growth in window chambers. Co-injection of erythropoietin antagonist proteins (soluble EPOR or anti-EPO antibody) with tumor cells or stable expression of antagonist R103A-EPO protein secreted from tumor cells inhibited angiogenesis and impaired tumor growth. In orthotopic tumor xenograft studies, EPOR-R129C expression significantly promoted tumor growth associated with increased expression of Ki67 proliferation antigen, enhanced microvessel density, decreased tumor hypoxia, and increased phosphorylation of extracellular-regulated kinases ERK1/2. R103A-EPO antagonist expression in mammary carcinoma cells was associated with near-complete disruption of primary tumor formation in the mammary fat pad.
Conclusions/significance: These data indicate that erythropoietin is an important angiogenic factor that regulates the induction of tumor cell-induced neovascularization and growth during the initial stages of tumorigenesis. The suppression of tumor angiogenesis and progression by erythropoietin blockade suggests that erythropoietin may constitute a potential target for the therapeutic modulation of angiogenesis in cancer.
Conflict of interest statement
Figures



References
-
- Huang Q, Shan S, Braun RD, Lanzen J, Anyrhambatla G, et al. Noninvasive visualization of tumors in rodent dorsal skin window chambers. Nat Biotechnol. 1999;17:1033–1035. - PubMed
-
- Li CY, Shan S, Huang Q, Braun RD, Lanzen J, et al. Initial stages of tumor cell-induced angiogenesis: evaluation via skin window chambers in rodent models. J Natl Cancer Inst. 2000;92:143–147. - PubMed
-
- Folkman J. Incipient angiogenesis. J Natl Cancer Inst. 2000;92:94–95. - PubMed
-
- Cao Y, Li CY, Moeller BJ, Yu D, Zhao Y, et al. Observation of incipient tumor angiogenesis that is independent of hypoxia and hypoxia inducible factor-1 activation. Cancer Res. 2005;65:5498–5505. - PubMed
-
- Shan S, Robson ND, Cao Y, Qiao T, Li CY, et al. Responses of vascular endothelial cells to angiogenic signaling are important for tumor cell survival. Faseb J. 2004;18:326–328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

