A bispecific diabody directed against prostate-specific membrane antigen and CD3 induces T-cell mediated lysis of prostate cancer cells
- PMID: 17579857
- PMCID: PMC2755730
- DOI: 10.1007/s00262-007-0348-6
A bispecific diabody directed against prostate-specific membrane antigen and CD3 induces T-cell mediated lysis of prostate cancer cells
Abstract
Background: Although cancer of the prostate is one of the most commonly diagnosed cancers in men, no curative treatment currently exists after its progression beyond resectable boundaries. Therefore, new agents for targeted treatment strategies are needed. Cross-linking of tumor antigens with T-cell associated antigens by bispecific monoclonal antibodies have been shown to increase antigen-specific cytotoxicity in T-cells. Since the prostate-specific membrane antigen (PSMA) represents an excellent tumor target, immunotherapy with bispecific diabodies could be a promising novel treatment option for prostate cancer.
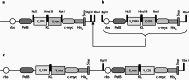
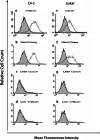
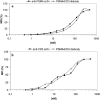
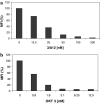
Methods: A heterodimeric diabody specific for human PSMA and the T-cell antigen CD3 was constructed from the DNA of anti-CD3 and anti-PSMA single chain Fv fragments (scFv). It was expressed in E. coli using a vector containing a bicistronic operon for co-secretion of the hybrid scFv V(H)CD3-V(L)PSMA and V(H)PSMA-V(L)CD3. The resulting PSMAxCD3 diabody was purified from the periplasmic extract by immobilized metal affinity chromatography (IMAC). The binding properties were tested on PSMA-expressing prostate cancer cells and PSMA-negative cell lines as well as on Jurkat cells by flow cytometry. For in vitro functional analysis, a cell viability test (WST) was used. For in vivo evaluation the diabody was applied together with human peripheral blood lymphocytes (PBL) in a C4-2 xenograft-SCID mouse model.
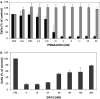
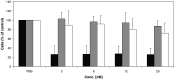
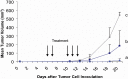
Results: By Blue Native gel electrophoresis, it could be shown that the PSMAxCD3 diabody is mainly a tetramer. Specific binding both to CD3-expressing Jurkat cells and PSMA-expressing C4-2 cells was shown by flow cytometry. In vitro, the diabody proved to be a potent agent for retargeting PBL to lyze C4-2 prostate cancer cells. Treatment of SCID mice inoculated with C4-2 tumor xenografts with the diabody and PBL efficiently inhibited tumor growth.
Conclusions: The PSMAxCD3 diabody bears the potential for facilitating immunotherapy of prostate cancer and for the elimination of minimal residual disease.
Figures

References
-
- Bohlen H, Hopff T, Manzke O, Engert A, Kube D, Wickramanayake PD, Diehl V, Tesch H. Lysis of malignant B cells from patients with B-chronic lymphocytic leukemia by autologous T cells activated with CD3 × CD19 bispecific antibodies in combination with bivalent CD28 antibodies. Blood. 1993;82:1803–1812. - PubMed
-
- Canevari S, Stoter G, Arienti F, Bolis G, Colnaghi MI, Di Re EM, Eggermont AM, Goey SH, Gratama JW, Lamers CH, et al. Regression of advanced ovarian carcinoma by intraperitoneal treatment with autologous T lymphocytes retargeted by a bispecific monoclonal antibody. J Natl Cancer Inst. 1995;87:1463–1469. doi: 10.1093/jnci/87.19.1463. - DOI - PubMed
-
- Chang SS, Reuter VE, Heston WD, Bander NH, Grauer LS, Gaudin PB. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999;59:3192–3198. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

