Long-term nightly treatment with indiplon in adults with primary insomnia: results of a double-blind, placebo-controlled, 3-month study
- PMID: 17580596
- PMCID: PMC1978349
- DOI: 10.1093/sleep/30.6.743
Long-term nightly treatment with indiplon in adults with primary insomnia: results of a double-blind, placebo-controlled, 3-month study
Abstract
Objectives: To evaluate the efficacy and safety of indiplon in primary insomnia.
Design: Randomized, double-blind, placebo-controlled, 3-month study.
Setting: Multi-center outpatient setting.
Patients: N=702 (61% female; mean age 46 years) who met DSM-IV criteria for primary insomnia of at least 3 months' duration.
Interventions: Indiplon 10 mg (n=236), indiplon 20 mg (n=233), or placebo (n=233).
Measurements: Subjective assessment of each of the following: latency to sleep onset (sLSO), total sleep time (sTST), number of awakenings after sleep onset (sNAASO), wake time after sleep onset (sWASO), sleep quality, Insomnia Severity Index (ISI), and global improvement.
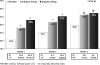
Results: Treatment with indiplon resulted in significant improvement relative to placebo at all time points for the primary endpoint, sLSO. Mean sLSO at Month 1 for each treatment group was: 10 mg (34.0 +/- 1.3 mins), 20 mg (33.0 +/- 1.3 mins), and placebo (48.7 +/- 1.9 mins; P <0.0001 for both comparisons); efficacy was sustained through Month 3. Both doses of indiplon resulted in significant improvement in sleep maintenance and duration endpoints, sTST and sWASO, as well as sleep quality, ISI, and global improvement at all assessment time points.
Conclusions: In patients with chronic insomnia, long-term nightly treatment with 10 mg and 20 mg doses of indiplon resulted in significant and sustained efficacy in sleep onset, maintenance, and duration, and significant associated improvement in both daytime functioning and quality of life.
Figures

References
-
- Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. - PubMed
-
- Janson C, Lindberg E, Gislason T, Elmasry A, Boman G. Insomnia in men: 10-year prospective population based study. Sleep. 2001;24:425–30. - PubMed
-
- Ohayon MM, Roth T. Place of chronic insomnia in the course of depressive and anxiety disorders. J Psychiatr Res. 2003;37:9–15. - PubMed
-
- Leger D, Guilleminault C, Bader G, Levy E, Paillard M. Medical and socio-professional impact of insomnia. Sleep. 2002;25:625–9. - PubMed
-
- Hatoum HT, Kong SX, Kania CM, Wong JM, Mendelson WB. Insomnia, health-related quality of life and healthcare resource consumption. A study of managed-care organisation enrollees. Pharmacoeconomics. 1998;14:629–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

