Determination of chondroitin sulfate content in raw materials and dietary supplements by high-performance liquid chromatography with ultraviolet detection after enzymatic hydrolysis: single-laboratory validation
- PMID: 17580617
- PMCID: PMC2614115
Determination of chondroitin sulfate content in raw materials and dietary supplements by high-performance liquid chromatography with ultraviolet detection after enzymatic hydrolysis: single-laboratory validation
Abstract
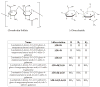
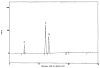
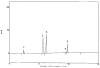
A method to quantify chondroitin sulfate in raw materials and dietary supplements at a range of about 5 to 100% (w/w) chondroitin sulfate has been developed and validated. The chondroitin sulfate is first selectively hydrolyzed by chondroitinase ACII enzyme to form un-, mono-, di-, and trisulfated unsaturated disaccharides; the resulting disaccharides are then quantified by ion-pairing liquid chromatography with ultraviolet detection. The amounts of the individual disaccharides are summed to yield the total amount of chondroitin sulfate in the material. Single-laboratory validation has been performed to determine the repeatability, accuracy, selectivity, limit of detection, limit of quantification, ruggedness, and linearity of the method. Repeatability precision for total chondroitin sulfate content was between 1.60 and 4.72% relative standard deviation, with HorRat values between 0.79 and 2.25. Chondroitin sulfate recovery from raw material negative control was between 101 and 102%, and recovery from finished product negative control was between 105 and 106%.
Figures


References
-
- Imanari T, Toida T, Koshiishi I, Toyoda H. J Chromatogr A. 1996;720:275–293. - PubMed
-
- Karamanos NK, Vanky P, Syrokou A, Hjerpe A. Anal Biochem. 1995;225:220–230. - PubMed
-
- Pelletier JP, Martel-Pelletier J, Mehraban F, Malemud CJ. J Orthopaed Res. 1992;10:511–523. - PubMed
-
- Malemud CJ, Papay RS, Hering TM, Holderbaum D, Goldberg VM, Haqqi TM. Osteoarthr Cartilage. 1995;3:227–238. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
