Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis
- PMID: 17581586
- PMCID: PMC2775137
- DOI: 10.1038/nature05883
Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis
Abstract
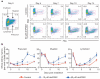
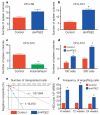
Haematopoietic stem cell (HSC) homeostasis is tightly controlled by growth factors, signalling molecules and transcription factors. Definitive HSCs derived during embryogenesis in the aorta-gonad-mesonephros region subsequently colonize fetal and adult haematopoietic organs. To identify new modulators of HSC formation and homeostasis, a panel of biologically active compounds was screened for effects on stem cell induction in the zebrafish aorta-gonad-mesonephros region. Here, we show that chemicals that enhance prostaglandin (PG) E2 synthesis increased HSC numbers, and those that block prostaglandin synthesis decreased stem cell numbers. The cyclooxygenases responsible for PGE2 synthesis were required for HSC formation. A stable derivative of PGE2 improved kidney marrow recovery following irradiation injury in the adult zebrafish. In murine embryonic stem cell differentiation assays, PGE2 caused amplification of multipotent progenitors. Furthermore, ex vivo exposure to stabilized PGE2 enhanced spleen colony forming units at day 12 post transplant and increased the frequency of long-term repopulating HSCs present in murine bone marrow after limiting dilution competitive transplantation. The conserved role for PGE2 in the regulation of vertebrate HSC homeostasis indicates that modulation of the prostaglandin pathway may facilitate expansion of HSC number for therapeutic purposes.
Figures

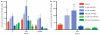
References
-
- Dzierzak E. The emergence of definitive hematopoietic stem cells in the mammal. Curr. Opin. Hematol. 2005;12:197–202. - PubMed
-
- Galloway JL, Zon LI. Ontogeny of hematopoiesis: examining the emergence of hematopoietic cells in the vertebrate embryo. Curr. Top. Dev. Biol. 2003;53:139–158. - PubMed
-
- North TE, et al. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity. 2002;16:661–672. - PubMed
-
- Mukouyama Y, et al. Hematopoietic cells in cultures of the murine embryonic aorta-gonad-mesonephros region are induced by c-Myb. Curr. Biol. 1999;9:833–836. - PubMed
-
- Kalev-Zylinska ML, et al. Runx1 is required for zebrafish blood and vessel development and expression of a human RUNX1-CBF2T1 transgene advances a model for studies of leukemogenesis. Development. 2002;129:2015–2030. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

