Identification of a VLDL-induced, FDNPVY-independent internalization mechanism for the LDLR
- PMID: 17581630
- PMCID: PMC1933400
- DOI: 10.1038/sj.emboj.7601769
Identification of a VLDL-induced, FDNPVY-independent internalization mechanism for the LDLR
Abstract
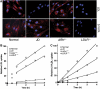
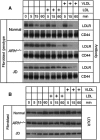
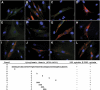
The low-density lipoprotein (LDL) receptor (LDLR) binds to and internalizes lipoproteins that contain apolipoproteinB100 (apoB100) or apolipoproteinE (apoE). Internalization of the apoB100 lipoprotein ligand, LDL, requires the FDNPVY(807) sequence on the LDLR cytoplasmic domain, which binds to the endocytic machinery of coated pits. We show here that inactivation of the FDNPVY(807) sequence by mutation of Y807 to cysteine prevented the uptake of LDL; however, this mutation did not prevent LDLR-dependent uptake of the apoE lipoprotein ligand, beta-VLDL. Comparison of the surface localization of the LDLR-Y807C using LDLR-immunogold, LDL-gold and beta-VLDL-gold probes revealed enrichment of LDLR-Y807C-bound beta-VLDL in coated pits, suggesting that beta-VLDL binding promoted the internalization of the LDLR-Y807C. Consistent with this possibility, treatment with monensin, which traps internalized LDLR in endosomes, resulted in the loss of surface LDLR-Y807C only when beta-VLDL was present. Reconstitution experiments in which LDLR variants were introduced into LDLR-deficient cells showed that the HIC(818) sequence is involved in beta-VLDL uptake by the LDLR-Y807C. Together, these experiments demonstrate that the LDLR has a very low-density lipoprotein (VLDL)-induced, FDNPVY-independent internalization mechanism.
Figures



References
-
- Anderson RG, Goldstein JL, Brown MS (1977) A mutation that impairs the ability of lipoprotein receptors to localise in coated pits on the cell surface of human fibroblasts. Nature 270: 695–699 - PubMed
-
- Arca M, Zuliani G, Wilund K, Campagna F, Fellin R, Bertolini S, Calandra S, Ricci G, Glorioso N, Maioli M, Pintus P, Carru C, Cossu F, Cohen J, Hobbs HH (2002) Autosomal recessive hypercholesterolaemia in Sardinia, Italy, and mutations in ARH: a clinical and molecular genetic analysis. Lancet 359: 841–847 - PubMed
-
- Basu SK, Goldstein JL, Anderson RG, Brown MS (1981) Monensin interrupts the recycling of low density lipoprotein receptors in human fibroblasts. Cell 24: 493–502 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

