Latent TGF-beta-binding protein 2 binds to DANCE/fibulin-5 and regulates elastic fiber assembly
- PMID: 17581631
- PMCID: PMC1933399
- DOI: 10.1038/sj.emboj.7601768
Latent TGF-beta-binding protein 2 binds to DANCE/fibulin-5 and regulates elastic fiber assembly
Abstract
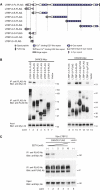
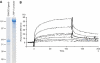
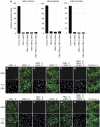
Elastic fibers play the principal roles in providing elasticity and integrity to various types of human organs, such as the arteries, lung, and skin. However, the molecular mechanism of elastic fiber assembly that leads to deposition and crosslinking of elastin along microfibrils remains largely unknown. We have previously shown that developing arteries and neural crest EGF-like protein (DANCE) (also designated fibulin-5) is essential for elastogenesis by studying DANCE-deficient mice. Here, we report the identification of latent transforming growth factor-beta-binding protein 2 (LTBP-2), an elastic fiber-associating protein whose function in elastogenesis is not clear, as a DANCE-binding protein. Elastogenesis assays using human skin fibroblasts reveal that fibrillar deposition of DANCE and elastin is largely dependent on fibrillin-1 microfibrils. However, downregulation of LTBP-2 induces fibrillin-1-independent fibrillar deposition of DANCE and elastin. Moreover, recombinant LTBP-2 promotes deposition of DANCE onto fibrillin-1 microfibrils. These results suggest a novel regulatory mechanism of elastic fiber assembly in which LTBP-2 regulates targeting of DANCE on suitable microfibrils to form elastic fibers.
Figures







Similar articles
-
Latent transforming growth factor beta-binding proteins and fibulins compete for fibrillin-1 and exhibit exquisite specificities in binding sites.J Biol Chem. 2009 Jun 19;284(25):16872-16881. doi: 10.1074/jbc.M809348200. Epub 2009 Apr 6. J Biol Chem. 2009. PMID: 19349279 Free PMC article.
-
LTBP-2 competes with tropoelastin for binding to fibulin-5 and heparin, and is a negative modulator of elastinogenesis.Matrix Biol. 2014 Feb;34:114-23. doi: 10.1016/j.matbio.2013.10.007. Epub 2013 Oct 19. Matrix Biol. 2014. PMID: 24148803
-
Assembly of fibrillin microfibrils governs extracellular deposition of latent TGF beta.J Cell Sci. 2010 Sep 1;123(Pt 17):3006-18. doi: 10.1242/jcs.073437. Epub 2010 Aug 10. J Cell Sci. 2010. PMID: 20699357 Free PMC article.
-
Recent updates on the molecular network of elastic fiber formation.Essays Biochem. 2019 Sep 13;63(3):365-376. doi: 10.1042/EBC20180052. Print 2019 Sep 13. Essays Biochem. 2019. PMID: 31395654 Review.
-
Fibrillin microfibrils and elastic fibre proteins: Functional interactions and extracellular regulation of growth factors.Semin Cell Dev Biol. 2019 May;89:109-117. doi: 10.1016/j.semcdb.2018.07.016. Epub 2018 Jul 20. Semin Cell Dev Biol. 2019. PMID: 30016650 Free PMC article. Review.
Cited by
-
Microfibril structure masks fibrillin-2 in postnatal tissues.J Biol Chem. 2010 Jun 25;285(26):20242-51. doi: 10.1074/jbc.M109.087031. Epub 2010 Apr 19. J Biol Chem. 2010. PMID: 20404337 Free PMC article.
-
Latent TGF-β binding protein-2 is essential for the development of ciliary zonule microfibrils.Hum Mol Genet. 2014 Nov 1;23(21):5672-82. doi: 10.1093/hmg/ddu283. Epub 2014 Jun 6. Hum Mol Genet. 2014. PMID: 24908666 Free PMC article.
-
Crossing Bridges between Extra- and Intra-Cellular Events in Thoracic Aortic Aneurysms.J Atheroscler Thromb. 2018 Feb 1;25(2):99-110. doi: 10.5551/jat.RV17015. Epub 2017 Sep 22. J Atheroscler Thromb. 2018. PMID: 28943527 Free PMC article. Review.
-
Dual functions for LTBP in lung development: LTBP-4 independently modulates elastogenesis and TGF-beta activity.J Cell Physiol. 2009 Apr;219(1):14-22. doi: 10.1002/jcp.21643. J Cell Physiol. 2009. PMID: 19016471 Free PMC article.
-
The role of fibrillin and microfibril binding proteins in elastin and elastic fibre assembly.Matrix Biol. 2019 Nov;84:17-30. doi: 10.1016/j.matbio.2019.06.006. Epub 2019 Jun 18. Matrix Biol. 2019. PMID: 31226403 Free PMC article. Review.
References
-
- Bailey AJ (2001) Molecular mechanisms of ageing in connective tissues. Mech Ageing Dev 122: 735–755 - PubMed
-
- Charbonneau NL, Dzamba BJ, Ono RN, Keene DR, Corson GM, Reinhardt DP, Sakai LY (2003) Fibrillins can co-assemble in fibrils, but fibrillin fibril composition displays cell-specific differences. J Biol Chem 278: 2740–2749 - PubMed
-
- Chaudhry SS, Gazzard J, Baldock C, Dixon J, Rock MJ, Skinner GC, Steel KP, Kielty CM, Dixon MJ (2001) Mutation of the gene encoding fibrillin-2 results in syndactyly in mice. Hum Mol Genet 10: 835–843 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

