A family of G protein βγ subunits translocate reversibly from the plasma membrane to endomembranes on receptor activation
- PMID: 17581822
- PMCID: PMC2238721
- DOI: 10.1074/jbc.M701191200
A family of G protein βγ subunits translocate reversibly from the plasma membrane to endomembranes on receptor activation
Abstract
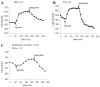
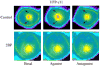
The present model of G protein activation by G protein-coupled receptors exclusively localizes their activation and function to the plasma membrane (PM). Observation of the spatiotemporal response of G protein subunits in a living cell to receptor activation showed that 6 of the 12 members of the G protein gamma subunit family translocate specifically from the PM to endomembranes. The gamma subunits translocate as betagamma complexes, whereas the alpha subunit is retained on the PM. Depending on the gamma subunit, translocation occurs predominantly to the Golgi complex or the endoplasmic reticulum. The rate of translocation also varies with the gamma subunit type. Different gamma subunits, thus, confer distinct spatiotemporal properties to translocation. A striking relationship exists between the amino acid sequences of various gamma subunits and their translocation properties. gamma subunits with similar translocation properties are more closely related to each other. Consistent with this relationship, introducing residues conserved in translocating subunits into a non-translocating subunit results in a gain of function. Inhibitors of vesicle-mediated trafficking and palmitoylation suggest that translocation is diffusion-mediated and controlled by acylation similar to the shuttling of G protein subunits (Chisari, M., Saini, D. K., Kalyanaraman, V., and Gautam, N. (2007) J. Biol. Chem. 282, 24092-24098). These results suggest that the continual testing of cytosolic surfaces of cell membranes by G protein subunits facilitates an activated cell surface receptor to direct potentially active G protein betagamma subunits to intracellular membranes.
Figures
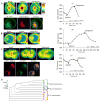
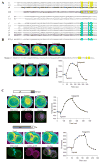
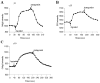
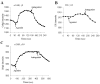
References
-
- Gilman AG. Annu Rev Biochem. 1987;56:615–649. - PubMed
-
- Simon MI, Strathmann MP, Gautam N. Science. 1991;252:802–808. - PubMed
-
- Neves SR, Ram PT, Iyengar R. Science. 2002;296:1636–1639. - PubMed
-
- Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, Hamm HE. Endocr Rev. 2003;24:765–781. - PubMed
-
- Kisselev O, Gautam N. J Biol Chem. 1993;268:24519–24522. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

