Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells
- PMID: 17581871
- PMCID: PMC2040893
- DOI: 10.1073/pnas.0703723104
Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells
Abstract
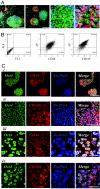
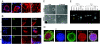
Control of self-renewal and differentiation of human ES cells (hESCs) remains a challenge. This is largely due to the use of culture systems that involve poorly defined animal products and do not mimic the normal developmental milieu. Routine protocols involve the propagation of hESCs on mouse fibroblast or human feeder layers, enzymatic cell removal, and spontaneous differentiation in cultures of embryoid bodies, and each of these steps involves significant variability of culture conditions. We report that a completely synthetic hydrogel matrix can support (i) long-term self-renewal of hESCs in the presence of conditioned medium from mouse embryonic fibroblast feeder layers, and (ii) direct cell differentiation. Hyaluronic acid (HA) hydrogels were selected because of the role of HA in early development and feeder layer cultures of hESCs and the controllability of hydrogel architecture, mechanics, and degradation. When encapsulated in 3D HA hydrogels (but not within other hydrogels or in monolayer cultures on HA), hESCs maintained their undifferentiated state, preserved their normal karyotype, and maintained their full differentiation capacity as indicated by embryoid body formation. Differentiation could be induced within the same hydrogel by simply altering soluble factors. We therefore propose that HA hydrogels, with their developmentally relevant composition and tunable physical properties, provide a unique microenvironment for the self-renewal and differentiation of hESCs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Science. 1998;282:1145–1147. - PubMed
-
- Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK. Nat Biotechnol. 2001;19:971–974. - PubMed
-
- Richards M, Fong CY, Chan WK, Wong PC, Bongso A. Nat Biotechnol. 2002;20:933–936. - PubMed
-
- Stoikovic P, Lako M, Przyborski S, Stewart R, Armstong L, Evans J, Zhang X, Stojkpvic M. Stem Cells. 2005;23:895–902. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

