Schwann cell-derived factors modulate synaptic activities at developing neuromuscular synapses
- PMID: 17581958
- PMCID: PMC6672697
- DOI: 10.1523/JNEUROSCI.1329-07.2007
Schwann cell-derived factors modulate synaptic activities at developing neuromuscular synapses
Abstract
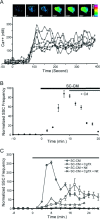
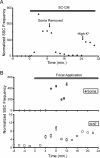
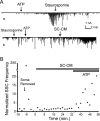
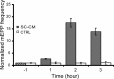
Glial cells are active participants in the function, formation, and maintenance of the chemical synapse. To investigate the molecular basis of neuron-glia interactions at the peripheral synapse, we examined whether and how Schwann cell-derived factors modulate synaptic function at developing neuromuscular junctions (NMJs). Schwann cell-conditioned medium (SC-CM) from Xenopus Schwann cell cultures was collected and applied to Xenopus nerve-muscle cocultures. We found that SC-CM increased the frequency of spontaneous synaptic currents (SSCs) within 3-15 min by an average of approximately 150-fold at developing neuromuscular synapses. The increase in SSC frequency by SC-CM is a presynaptic effect independent of neuronal excitability and requires the influx of Ca2+. In contrast to its potentiating effect on spontaneous transmitter release, SC-CM suppressed the evoked transmitter release. The SC-CM effect required the presence of motoneuron soma but not protein synthesis. Using molecular weight cutoff filters and dialysis membranes, we found that the molecular weight of functional factor(s) in SC-CM was within 500 and 5000 Da. The SC-CM effect was not attributable to currently known factors that modulate synaptic efficacy, including neurotrophins, glutamate, and ATP. SC-CM also enhanced spontaneous synaptic release at developing NMJs in Xenopus tadpoles in situ. Our results suggest that Schwann cells release small molecules that enhance spontaneous synaptic activities acutely and potently at developing neuromuscular synapses, and the glial cell-enhanced spontaneous neurotransmission may contribute to synaptogenesis.
Figures



References
-
- Allen NJ, Barres BA. Signaling between glia and neurons: focus on synaptic plasticity. Curr Opin Neurobiol. 2005;15:542–548. - PubMed
-
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. - PubMed
-
- Astrow SH, Qiang H, Ko CP. Perisynaptic Schwann cells at neuromuscular junctions revealed by a novel monoclonal antibody. J Neurocytol. 1998;27:667–681. - PubMed
-
- Auld DS, Robitaille R. Perisynaptic Schwann cells at the neuromuscular junction: nerve- and activity-dependent contributions to synaptic efficacy, plasticity, and reinnervation. The Neuroscientist. 2003;9:144–157. - PubMed
-
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
