NKCC1 phosphorylation stimulates neurite growth of injured adult sensory neurons
- PMID: 17581962
- PMCID: PMC6672700
- DOI: 10.1523/JNEUROSCI.1337-07.2007
NKCC1 phosphorylation stimulates neurite growth of injured adult sensory neurons
Abstract
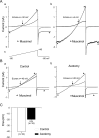
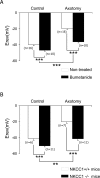
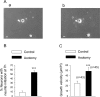
Peripheral nerve section promotes regenerative, elongated neuritic growth of adult sensory neurons. Although the role of chloride homeostasis, through the regulation of ionotropic GABA receptors, in the growth status of immature neurons in the CNS begins to emerge, nothing is known of its role in the regenerative growth of injured adult neurons. To analyze the intracellular Cl- variation after a sciatic nerve section in vivo, gramicidin perforated-patch recordings were used to study muscimol-induced currents in mice dorsal root ganglion neurons isolated from control and axotomized neurons. We show that the reversal potential of muscimol-induced current, E(GABA-A), was shifted toward depolarized potentials in axotomized neurons. This was attributable to Cl- influx because removal of extracellular Cl- prevented this shift. Application of bumetanide, an inhibitor of NKCC1 cotransporter and E(GABA-A) recordings in sensory neurons from NKCC1-/- mice, identified NKCC1 as being responsible for the increase in intracellular Cl- in axotomized neurons. In addition, we demonstrate with a phospho-NKCC1 antibody that nerve injury induces an increase in the phosphorylated form of NKCC1 in dorsal root ganglia that could account for intracellular Cl- accumulation. Time-lapse recordings of the neuritic growth of axotomized neurons show a faster growth velocity compared with control. Bumetanide, the intrathecal injection of NKCC1 small interfering RNA, and the use of NKCC1-/- mice demonstrated that NKCC1 is involved in determining the velocity of elongated growth of axotomized neurons. Our results clearly show that NKCC1-induced increase in intracellular chloride concentration is a major event accompanying peripheral nerve regeneration.
Figures




References
-
- Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB, Levine JD. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron. 2003;39:497–511. - PubMed
-
- Alvarez-Leefmans FJ, Leon-Olea M, Mendoza-Sotelo J, Alvarez FJ, Anton B, Garduno R. Immunolocalization of the Na+-K+-2Cl− cotransporter in peripheral nervous tissue of vertebrates. Neuroscience. 2001;104:569–582. - PubMed
-
- Andre S, Boukhaddaoui H, Campo B, Al-Jumaily M, Mayeux V, Greuet D, Valmier J, Scamps F. Axotomy-induced expression of calcium-activated chloride current in subpopulations of mouse dorsal root ganglion neurons. J Neurophysiol. 2003;90:3764–3773. - PubMed
-
- Araki T, Nagarajan R, Milbrandt J. Identification of genes induced in peripheral nerve after injury. Expression profiling and novel gene discovery. J Biol Chem. 2001;276:34131–34141. - PubMed
-
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
