Alzheimer's-type amyloidosis in transgenic mice impairs survival of newborn neurons derived from adult hippocampal neurogenesis
- PMID: 17581964
- PMCID: PMC4439193
- DOI: 10.1523/JNEUROSCI.5564-06.2007
Alzheimer's-type amyloidosis in transgenic mice impairs survival of newborn neurons derived from adult hippocampal neurogenesis
Abstract
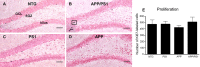
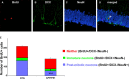
Alzheimer's disease (AD) is characterized by severe neuronal loss in several brain regions important for learning and memory. Of the structures affected by AD, the hippocampus is unique in continuing to produce new neurons throughout life. Mounting evidence indicates that hippocampal neurogenesis contributes to the processing and storage of new information and that deficits in the production of new neurons may impair learning and memory. Here, we examine whether the overproduction of amyloid-beta (Abeta) peptide in a mouse model for AD might be detrimental to newborn neurons in the hippocampus. We used transgenic mice overexpressing familial AD variants of amyloid precursor protein (APP) and/or presenilin-1 to test how the level (moderate or high) and the aggregation state (soluble or deposited) of Abeta impacts the proliferation and survival of new hippocampal neurons. Although proliferation and short-term survival of neural progenitors in the hippocampus was unaffected by APP/Abeta overproduction, survival of newborn cells 4 weeks later was dramatically diminished in transgenic mice with Alzheimer's-type amyloid pathology. Phenotypic analysis of the surviving population revealed a specific reduction in newborn neurons. Our data indicate that overproduction of Abeta and the consequent appearance of amyloid plaques cause an overall reduction in the number of adult-generated hippocampal neurons. Diminished capacity for hippocampal neuron replacement may contribute to the cognitive decline observed in these mice.
Figures




References
-
- Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. - PubMed
-
- Arendash GW, Garcia MF, Costa DA, Cracchiolo JR, Wefes IM, Potter H. Environmental enrichment improves cognition in aged Alzheimer's transgenic mice despite stable beta-amyloid deposition. NeuroReport. 2004;15:1751–1754. - PubMed
-
- Bell KF, Ducatenzeiler A, Ribeiro-da-Silva A, Duff K, Bennett DA, Claudio Cuello A. The amyloid pathology progresses in a neurotransmitter-specific manner. Neurobiol Aging. 2006;27:1644–1657. - PubMed
-
- Benzing WC, Wujek JR, Ward EK, Shaffer D, Ashe KH, Younkin SG, Brunden KR. Evidence for glial-mediated inflammation in aged APP(SW) transgenic mice. Neurobiol Aging. 1999;20:581–589. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
