An RNA pseudoknot in the 3' end of the arterivirus genome has a critical role in regulating viral RNA synthesis
- PMID: 17581985
- PMCID: PMC1951461
- DOI: 10.1128/JVI.00747-07
An RNA pseudoknot in the 3' end of the arterivirus genome has a critical role in regulating viral RNA synthesis
Abstract
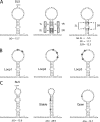
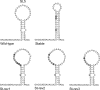
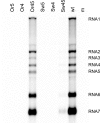
In the life cycle of plus-strand RNA viruses, the genome initially serves as the template for both translation of the viral replicase gene and synthesis of minus-strand RNA and is ultimately packaged into progeny virions. These various processes must be properly balanced to ensure efficient viral proliferation. To achieve this, higher-order RNA structures near the termini of a variety of RNA virus genomes are thought to play a key role in regulating the specificity and efficiency of viral RNA synthesis. In this study, we have analyzed the signals for minus-strand RNA synthesis in the prototype of the arterivirus family, equine arteritis virus (EAV). Using site-directed mutagenesis and an EAV reverse genetics system, we have demonstrated that a stem-loop structure near the 3' terminus of the EAV genome is required for RNA synthesis. We have also obtained evidence for an essential pseudoknot interaction between the loop region of this stem-loop structure and an upstream hairpin residing in the gene encoding the nucleocapsid protein. We propose that the formation of this pseudoknot interaction may constitute a molecular switch that could regulate the specificity or timing of viral RNA synthesis. This hypothesis is supported by the fact that phylogenetic analysis predicted the formation of similar pseudoknot interactions near the 3' end of all known arterivirus genomes, suggesting that this interaction has been conserved in evolution.
Figures


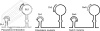

References
-
- Beerens, N., and E. J. Snijder. 2006. RNA signals in the 3′ terminus of the genome of equine arteritis virus are required for viral RNA synthesis. J. Gen. Virol. 87:1977-1983. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

