Arenavirus Z-glycoprotein association requires Z myristoylation but not functional RING or late domains
- PMID: 17581989
- PMCID: PMC1951451
- DOI: 10.1128/JVI.00499-07
Arenavirus Z-glycoprotein association requires Z myristoylation but not functional RING or late domains
Abstract
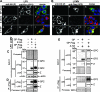
Generation of infectious arenavirus-like particles requires the virus RING finger Z protein and surface glycoprotein precursor (GPC) and the correct processing of GPC into GP1, GP2, and a stable signal peptide (SSP). Z is the driving force of arenavirus budding, whereas the GP complex (GPc), consisting of hetero-oligomers of SSP, GP1, and GP2, forms the viral envelope spikes that mediate receptor recognition and cell entry. Based on the roles played by Z and GP in the arenavirus life cycle, we hypothesized that Z and the GPc should interact in a manner required for virion formation. Here, using confocal microscopy and coimmunoprecipitation assays, we provide evidence for subcellular colocalization and biochemical interaction, respectively, of Z and the GPc. Our results from mutation-function analysis reveal that Z myristoylation, but not the Z late (L) or RING domain, is required for Z-GPc interaction. Moreover, Z interacted directly with SSP in the absence of other components of the GPc. We obtained similar results with Z and GPC from the prototypical arenavirus lymphocytic choriomeningitis virus and the hemorrhagic fever arenavirus Lassa fever virus.
Figures





References
-
- Aizaki, H., K. J. Lee, V. M. Sung, H. Ishiko, and M. M. Lai. 2004. Characterization of the hepatitis C virus RNA replication complex associated with lipid rafts. Virology 324:450-461. - PubMed
-
- Barton, L. L., M. B. Mets, and C. L. Beauchamp. 2002. Lymphocytic choriomeningitis virus: emerging fetal teratogen. Am. J. Obstet. Gynecol. 187:1715-1716. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

