The HBV drug entecavir - effects on HIV-1 replication and resistance
- PMID: 17582071
- PMCID: PMC3069686
- DOI: 10.1056/NEJMoa067710
The HBV drug entecavir - effects on HIV-1 replication and resistance
Abstract
Entecavir, a drug approved by the Food and Drug Administration for the treatment of chronic hepatitis B virus (HBV) infection, is not believed to inhibit replication of human immunodeficiency virus type 1 (HIV-1) at clinically relevant doses. We observed that entecavir led to a consistent 1-log(10) decrease in HIV-1 RNA in three persons with HIV-1 and HBV coinfection, and we obtained supportive in vitro evidence that entecavir is a potent partial inhibitor of HIV-1 replication. Detailed analysis showed that in one of these patients, entecavir monotherapy led to an accumulation of HIV-1 variants with the lamivudine-resistant mutation, M184V. In vitro experiments showed that M184V confers resistance to entecavir. Until more is known about HIV-1-resistance patterns and their selection by entecavir, caution is needed with the use of entecavir in persons with HIV-1 and HBV coinfection who are not receiving fully suppressive antiretroviral regimens.
Copyright 2007 Massachusetts Medical Society.
Figures
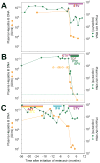
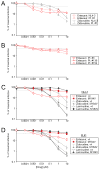
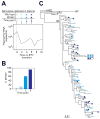
Comment in
-
Entecavir surprise.N Engl J Med. 2007 Jun 21;356(25):2641-3. doi: 10.1056/NEJMe078045. N Engl J Med. 2007. PMID: 17582076 No abstract available.
-
The anti-HIV antiviral activity of entecavir: the loss of a trusted friend?J Hepatol. 2007 Dec;47(6):872-4. doi: 10.1016/j.jhep.2007.09.002. Epub 2007 Oct 1. J Hepatol. 2007. PMID: 17931735
References
-
- Heijtink RA, De Wilde GA, Kruining J, et al. Inhibitory effect of 9-(2-phosphonylmethoxyethyl)-adenine (PMEA) on human and duck hepatitis B virus infection. Antiviral Res. 1993;21(2):141–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
