Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2
- PMID: 17582332
- PMCID: PMC3738748
- DOI: 10.1016/j.neuron.2007.05.029
Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2
Abstract
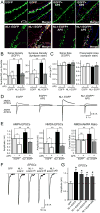
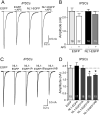
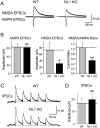
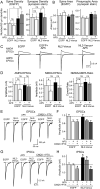
Neuroligins enhance synapse formation in vitro, but surprisingly are not required for the generation of synapses in vivo. We now show that in cultured neurons, neuroligin-1 overexpression increases excitatory, but not inhibitory, synaptic responses, and potentiates synaptic NMDAR/AMPAR ratios. In contrast, neuroligin-2 overexpression increases inhibitory, but not excitatory, synaptic responses. Accordingly, deletion of neuroligin-1 in knockout mice selectively decreases the NMDAR/AMPAR ratio, whereas deletion of neuroligin-2 selectively decreases inhibitory synaptic responses. Strikingly, chronic inhibition of NMDARs or CaM-Kinase II, which signals downstream of NMDARs, suppresses the synapse-boosting activity of neuroligin-1, whereas chronic inhibition of general synaptic activity suppresses the synapse-boosting activity of neuroligin-2. Taken together, these data indicate that neuroligins do not establish, but specify and validate, synapses via an activity-dependent mechanism, with different neuroligins acting on distinct types of synapses. This hypothesis reconciles the overexpression and knockout phenotypes and suggests that neuroligins contribute to the use-dependent formation of neural circuits.
Figures



References
-
- Agmon A, Connors BW. Thalamocortical response of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41:365–379. - PubMed
-
- Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, Sudhof TC. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. - PubMed
-
- Boucard AA, Chubykin AA, Comoletti D, Taylor P, Sudhof TC. A Splice Code for trans-Synaptic Cell Adhesion Mediated by Binding of NL1 to alpha- and beta-Neurexins. Neuron. 2005;48:229–236. - PubMed
-
- Cantallops I, Cline HT. Synapse formation: if it looks like a duck and quacks like a duck. Curr Biol. 2000;10:R620–623. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

