Role of the vasodilator peptide angiotensin-(1-7) in cardiovascular drug therapy
- PMID: 17583183
- PMCID: PMC1994039
Role of the vasodilator peptide angiotensin-(1-7) in cardiovascular drug therapy
Abstract
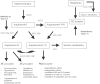
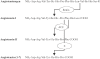
The renin-angiotensin-system (RAS) is a cascade of enzymatic reactions resulting ultimately in the formation of angiotensin II. Recent research has expanded the knowledge about the RAS by adding new components to the pathways: angiotensin-(1-5) [Ang-1-5], angiotensin-(1-7) [Ang-(1-7)], angiotensin-(1-9) [Ang-(1-9)], an ACE homologous enzyme, ACE2, and the G-protein-coupled receptor mas as a molecular receptor for Ang-(1-7). Although previous studies provided some conflicting evidence about the relevance of Ang-(1-7) in the regulation of vascular and renal function, data now demonstrate that Ang-(1-7) contributes to the cardiovascular effects of ACE-inhibitors (ACE-1) and AT1-receptor-blockers (ARBs) both in experimental conditions and in humans. This review summarizes and critically discusses the currently available experimental and clinical study evidence for the role of Ang-(1-7) as a vasodilator and anti-trophic peptide in cardiovascular drug therapy. In addition, the potential therapeutic impact of currently available RAS blocking agents (ACE-1 and ARBs) and new agents still under development (renin-inhibitors) on the RAS-effector peptides is highlighted.
Figures
Similar articles
-
The vasoprotective axes of the renin-angiotensin system: Physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases.Pharmacol Res. 2017 Nov;125(Pt A):21-38. doi: 10.1016/j.phrs.2017.06.005. Epub 2017 Jun 12. Pharmacol Res. 2017. PMID: 28619367 Free PMC article. Review.
-
Activation of the MAS receptor by angiotensin-(1-7) in the renin-angiotensin system mediates mesenteric vasodilatation in cirrhosis.Gastroenterology. 2013 Oct;145(4):874-884.e5. doi: 10.1053/j.gastro.2013.06.036. Epub 2013 Jun 22. Gastroenterology. 2013. PMID: 23796456
-
Recent insights and therapeutic perspectives of angiotensin-(1-9) in the cardiovascular system.Clin Sci (Lond). 2014 Nov;127(9):549-57. doi: 10.1042/CS20130449. Clin Sci (Lond). 2014. PMID: 25029123 Review.
-
Angiotensin-(1-7): beyond the cardio-renal actions.Clin Sci (Lond). 2013 Apr;124(7):443-56. doi: 10.1042/CS20120461. Clin Sci (Lond). 2013. PMID: 23249272 Review.
-
Angiotensin-(1-7) as a strategy in the treatment of hypertension?Curr Opin Nephrol Hypertens. 2014 Sep;23(5):480-6. doi: 10.1097/MNH.0000000000000050. Curr Opin Nephrol Hypertens. 2014. PMID: 25023950 Review.
Cited by
-
Alamandine alleviated heart failure and fibrosis in myocardial infarction mice.Biol Direct. 2022 Sep 27;17(1):25. doi: 10.1186/s13062-022-00338-6. Biol Direct. 2022. PMID: 36167556 Free PMC article.
-
The Impact of microRNAs in Renin-Angiotensin-System-Induced Cardiac Remodelling.Int J Mol Sci. 2021 Apr 30;22(9):4762. doi: 10.3390/ijms22094762. Int J Mol Sci. 2021. PMID: 33946230 Free PMC article. Review.
-
ACE2: more of Ang-(1-7) or less Ang II?Curr Opin Nephrol Hypertens. 2011 Jan;20(1):1-6. doi: 10.1097/MNH.0b013e3283406f57. Curr Opin Nephrol Hypertens. 2011. PMID: 21045683 Free PMC article. Review.
-
Local Renin-Angiotensin system in the reproductive system.Front Endocrinol (Lausanne). 2013 Oct 18;4:150. doi: 10.3389/fendo.2013.00150. Front Endocrinol (Lausanne). 2013. PMID: 24151488 Free PMC article. Review.
-
Angiotensin-Converting Enzyme 2 in the Pathogenesis of Renal Abnormalities Observed in COVID-19 Patients.Front Physiol. 2021 Aug 23;12:700220. doi: 10.3389/fphys.2021.700220. eCollection 2021. Front Physiol. 2021. PMID: 34497535 Free PMC article. Review.
References
-
- Abbas A, Gorelik G, Carbini LA, et al. Angiotensin-(1-7) induces bradykinin-mediated hypotensive responses in anesthetized rats. Hypertension. 1997;30:217–21. - PubMed
-
- Admiraal PJ, Derkx FH, Danser AH, et al. Metabolism and production of angiotensin I in different vascular beds in subjects with hypertension. Hypertension. 1990;15:44–55. - PubMed
-
- Almeida AP, Frabregas BC, Madureira MM, et al. Angiotensin-(1–7) potentiates the coronary vasodilatatory effect of bradykinin in the isolated rat heart. Braz J Med Biol Res. 2000;33:709–13. - PubMed
-
- Ambuhl P, Felix D, Khosla MC. [7-D-ALA]-angiotensin-(1–7): selective antagonism of angiotensin-(1–7) in the rat paraventricular nucleus. Brain Res Bull. 1994;35:289–91. - PubMed
-
- Andreatta-Van Leyen S, Romero MF, Khosla MC, et al. Modulation of phospholipase A2-activity and sodium transport by angiotensin-(1–7) Kidney Int. 1993;44:932–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

