Congenital muscular dystrophies involving the O-mannose pathway
- PMID: 17584082
- PMCID: PMC2855644
- DOI: 10.2174/156652407780831601
Congenital muscular dystrophies involving the O-mannose pathway
Abstract
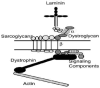
A number of forms of congenital muscular dystrophy (CMD) have been identified that involve defects in the glycosylation of dystroglycan with O-mannosyl-linked glycans. There are at least six genes that can affect this type of glycosylation, and defects in these genes give rise to disorders that have many aspects of muscle and brain pathology in common. Overexpression of one gene implicated in CMD, LARGE, was recently shown to increase dystroglycan glycosylation and restore its function in cells taken from CMD patients. Overexpression of Galgt2, a glycosyltransferase not implicated in CMD, also alters dystroglycan glycosylation and inhibits muscular dystrophy in a mouse model of Duchenne muscular dystrophy. These findings suggest that a common approach to therapy in muscular dystrophies may be to increase the glycosylation of dystroglycan with particular glycan structures.
Figures

References
-
- Freeze HH. Curr Mol Med. 2007;7(4):???–???. - PubMed
-
- Martin PT, Freeze HH. Glycobiology. 2003;13:67R–75R. - PubMed
-
- Michele DE, Campbell KP. J Biol Chem. 2003;278:15457–15460. - PubMed
-
- Muntoni F, Brockington M, Torelli S, Brown SC. Curr Opin Neurol. 2004;17:205–209. - PubMed
-
- Endo T, Toda T. Biol Pharm Bull. 2003;26:1641–1647. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
