Flow and myocardial interaction: an imaging perspective
- PMID: 17584731
- PMCID: PMC2440399
- DOI: 10.1098/rstb.2007.2119
Flow and myocardial interaction: an imaging perspective
Abstract
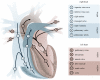
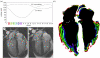
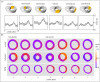
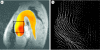
Heart failure due to coronary artery disease has considerable morbidity and poor prognosis. An understanding of the underlying mechanics governing myocardial contraction is a prerequisite for interpreting and predicting changes induced by heart disease. Gross changes in contractile behaviour of the myocardium are readily detected with existing techniques. For more subtle changes during early stages of cardiac dysfunction, however, a sensitive method for measuring, as well as a precise criterion for quantifying, normal and impaired myocardial function is required. The purpose of this paper is to outline the role of imaging, particularly cardiovascular magnetic resonance (CMR), for investigating the fundamental relationships between cardiac morphology, function and flow. CMR is emerging as an important clinical tool owing to its safety, versatility and the high-quality images it produces that allow accurate and reproducible quantification of cardiac structure and function. We demonstrate how morphological and functional assessment of the heart can be achieved by CMR and illustrate how blood flow imaging can be used to study flow and structure interaction, particularly for elucidating the underlying haemodynamic significance of directional changes and asymmetries of the cardiac looping. Future outlook on combining imaging with engineering approaches in subject-specific biomechanical simulation is also provided.
Figures


References
-
- Augst D, Barratt D, Hughes A, Glor F, Thom S, Xu X. Accuracy and reproducibility of CFD predicted wall shear stress using 3D ultrasound images. ASME J. Biomed. Eng. 2003;125:218–222. doi:10.1115/1.1553973 - DOI - PubMed
-
- Aurigemma G, Reichek N, Schiebler M, Axel L. Evaluation of mitral regurgitation by cine magnetic resonance imaging. Am. J. Cardiol. 1990;66:621–625. doi:10.1016/0002-9149(90)90491-I - DOI - PubMed
-
- Axel L, Dougherty L. MR imaging of motion with spatial modulation of magnetisation. Radiology. 1989;171:841–845. - PubMed
-
- Berne R.M. The heart. vol. 1. American Physiological Society; Bethesda, MD: 1979. Handbook of physiology: a critical, comprehensive presentation of physiological knowledge and concepts. Section 2: the cardiovascular system.
-
- Bogren H.G, Buonocore M.H. 4D magnetic resonance velocity mapping of blood flow patterns in the aorta in young vs elderly normal subjects. J. Magn. Reson. Imaging. 1999;10:861–869. doi:10.1002/(SICI)1522-2586(199911)10:5<861::AID-JMRI35>3.0.CO;2-E - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
