Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization
- PMID: 17584831
- PMCID: PMC2277224
- DOI: 10.1113/jphysiol.2007.133231
Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization
Abstract
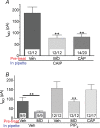
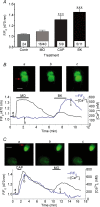
The pharmacological desensitization of receptors is a fundamental mechanism for regulating the activity of neuronal systems. The TRPA1 channel plays a key role in the processing of noxious information and can undergo functional desensitization by unknown mechanisms. Here we show that TRPA1 is desensitized by homologous (mustard oil; a TRPA1 agonist) and heterologous (capsaicin; a TRPV1 agonist) agonists via Ca2+-independent and Ca2+-dependent pathways, respectively, in sensory neurons. The pharmacological desensitization of TRPA1 by capsaicin and mustard oil is not influenced by activation of protein phosphatase 2B. However, it is regulated by phosphatidylinositol-4,5-bisphosphate depletion after capsaicin, but not mustard oil, application. Using a biosensor, we establish that capsaicin, unlike mustard oil, consistently activates phospholipase C in sensory neurons. We next demonstrate that TRPA1 desensitization is regulated by TRPV1, and it appears that mustard oil-induced TRPA1 internalization is prevented by coexpression with TRPV1 in a heterologous expression system and in sensory neurons. In conclusion, we propose novel mechanisms whereby TRPA1 activity undergoes pharmacological desensitization through multiple cellular pathways that are agonist dependent and modulated by TRPV1.
Figures
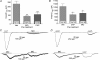






References
-
- Abe J, Hosokawa H, Sawada Y, Matsumura K, Kobayashi S. Ca2+-dependent PKC activation mediates menthol-induced desensitization of transient receptor potential M8. Neurosci Lett. 2006;397:140–144. - PubMed
-
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. - PubMed
-
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. - PubMed
-
- Bernstein GM, Jones OT. Kinetics of internalization and degradation of N-type voltage-gated calcium channels: Role of the α2/δ subunit. Cell Calcium. 2006;41:27–40. - PubMed
-
- Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWT. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

