Acute exacerbations of idiopathic pulmonary fibrosis
- PMID: 17585107
- PMCID: PMC2094133
- DOI: 10.1164/rccm.200703-463PP
Acute exacerbations of idiopathic pulmonary fibrosis
Abstract
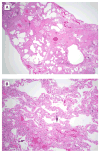
The natural history of idiopathic pulmonary fibrosis (IPF) has been characterized as a steady, predictable decline in lung function over time. Recent evidence suggests that some patients may experience a more precipitous course, with periods of relative stability followed by acute deteriorations in respiratory status. Many of these acute deteriorations are of unknown etiology and have been termed acute exacerbations of IPF. This perspective is the result of an international effort to summarize the current state of knowledge regarding acute exacerbations of IPF. Acute exacerbations of IPF are defined as acute, clinically significant deteriorations of unidentifiable cause in patients with underlying IPF. Proposed diagnostic criteria include subjective worsening over 30 days or less, new bilateral radiographic opacities, and the absence of infection or another identifiable etiology. The potential pathobiological roles of infection, disordered cell biology, coagulation, and genetics are discussed, and future research directions are proposed.
Conflict of interest statement
Figures

Comment in
-
Basis of acute exacerbation of idiopathic pulmonary fibrosis in Japanese patients.Am J Respir Crit Care Med. 2008 Jun 15;177(12):1397-8; author reply 1398. doi: 10.1164/ajrccm.177.12.1397a. Am J Respir Crit Care Med. 2008. PMID: 18522953 No abstract available.
-
An algorithm to tackle acute exacerbations in idiopathic pulmonary fibrosis.Am J Respir Crit Care Med. 2008 Jun 15;177(12):1397; author reply 1398. doi: 10.1164/ajrccm.177.12.1397. Am J Respir Crit Care Med. 2008. PMID: 18522954 No abstract available.
References
-
- American Thoracic Society/European Respiratory Society. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2002;165:277–304. - PubMed
-
- American Thoracic Society/European Respiratory Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. Am J Respir Crit Care Med. 2000;161:646–664. - PubMed
-
- Bjoraker JA, Ryu JH, Edwin MK, Myers JL, Tazelaar HD, Schroeder DR, Offord KP. Prognostic significance of histopathologic subsets in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1998;157:199–203. - PubMed
-
- Flaherty KR, Travis WD, Colby TV, Toews GB, Kazerooni EA, Gross BH, Jain A, Strawderman RL, Flint A, Lynch JP, et al. Histopathologic variability in usual and nonspecific interstitial pneumonias. Am J Respir Crit Care Med. 2001;164:1722–1727. - PubMed
-
- Nicholson AG, Colby TV, Dubois RM, Hansell DM, Wells AU. The prognostic significance of the histologic pattern of interstitial pneumonia in patients presenting with the clinical entity of cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med. 2000;162:2213–2217. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

