Sensory afferents regenerated into dorsal columns after spinal cord injury remain in a chronic pathophysiological state
- PMID: 17585905
- PMCID: PMC3103885
- DOI: 10.1016/j.expneurol.2007.05.013
Sensory afferents regenerated into dorsal columns after spinal cord injury remain in a chronic pathophysiological state
Abstract
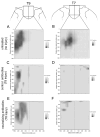
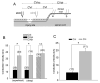
Axon regeneration after experimental spinal cord injury (SCI) can be promoted by combinatorial treatments that increase the intrinsic growth capacity of the damaged neurons and reduce environmental factors that inhibit axon growth. A prior peripheral nerve conditioning lesion is a well-established means of increasing the intrinsic growth state of sensory neurons whose axons project within the dorsal columns of the spinal cord. Combining such a prior peripheral nerve conditioning lesion with the infusion of antibodies that neutralize the growth inhibitory effects of the NG2 chondroitin sulfate proteoglycan promotes sensory axon growth through the glial scar and into the white matter of the dorsal columns. The physiological properties of these regenerated axons, particularly in the chronic SCI phase, have not been established. Here we examined the functional status of regenerated sensory afferents in the dorsal columns after SCI. Six months post-injury, we located and electrically mapped functional sensory axons that had regenerated beyond the injury site. The regenerated axons had reduced conduction velocity, decreased frequency-following ability, and increasing latency to repetitive stimuli. Many of the axons that had regenerated into the dorsal columns rostral to the injury site were chronically demyelinated. These results demonstrate that regenerated sensory axons remain in a chronic pathophysiological state and emphasize the need to restore normal conduction properties to regenerated axons after spinal cord injury.
Figures
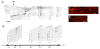


Similar articles
-
Antibodies against the NG2 proteoglycan promote the regeneration of sensory axons within the dorsal columns of the spinal cord.J Neurosci. 2006 May 3;26(18):4729-39. doi: 10.1523/JNEUROSCI.3900-05.2006. J Neurosci. 2006. PMID: 16672645 Free PMC article.
-
Expressing Constitutively Active Rheb in Adult Neurons after a Complete Spinal Cord Injury Enhances Axonal Regeneration beyond a Chondroitinase-Treated Glial Scar.J Neurosci. 2015 Aug 5;35(31):11068-80. doi: 10.1523/JNEUROSCI.0719-15.2015. J Neurosci. 2015. PMID: 26245968 Free PMC article.
-
Competition with Primary Sensory Afferents Drives Remodeling of Corticospinal Axons in Mature Spinal Motor Circuits.J Neurosci. 2016 Jan 6;36(1):193-203. doi: 10.1523/JNEUROSCI.3441-15.2016. J Neurosci. 2016. PMID: 26740661 Free PMC article.
-
Spinal cord regeneration.Cell Transplant. 2014;23(4-5):573-611. doi: 10.3727/096368914X678427. Cell Transplant. 2014. PMID: 24816452 Review.
-
Cortical and subcortical plasticity in the brains of humans, primates, and rats after damage to sensory afferents in the dorsal columns of the spinal cord.Exp Neurol. 2008 Feb;209(2):407-16. doi: 10.1016/j.expneurol.2007.06.014. Epub 2007 Jul 6. Exp Neurol. 2008. PMID: 17692844 Free PMC article. Review.
Cited by
-
Cortical and Subcortical Effects of Transcutaneous Spinal Cord Stimulation in Humans with Tetraplegia.J Neurosci. 2020 Mar 25;40(13):2633-2643. doi: 10.1523/JNEUROSCI.2374-19.2020. Epub 2020 Jan 29. J Neurosci. 2020. PMID: 31996455 Free PMC article.
-
Neutralization of inhibitory molecule NG2 improves synaptic transmission, retrograde transport, and locomotor function after spinal cord injury in adult rats.J Neurosci. 2013 Feb 27;33(9):4032-43. doi: 10.1523/JNEUROSCI.4702-12.2013. J Neurosci. 2013. PMID: 23447612 Free PMC article.
-
Early withdrawal of axons from higher centers in response to peripheral somatosensory denervation.J Neurosci. 2009 Mar 25;29(12):3738-48. doi: 10.1523/JNEUROSCI.5388-08.2009. J Neurosci. 2009. PMID: 19321770 Free PMC article.
-
A novel growth-promoting pathway formed by GDNF-overexpressing Schwann cells promotes propriospinal axonal regeneration, synapse formation, and partial recovery of function after spinal cord injury.J Neurosci. 2013 Mar 27;33(13):5655-67. doi: 10.1523/JNEUROSCI.2973-12.2013. J Neurosci. 2013. PMID: 23536080 Free PMC article.
-
Human neural stem cells differentiate and promote locomotor recovery in an early chronic spinal cord injury NOD-scid mouse model.PLoS One. 2010 Aug 18;5(8):e12272. doi: 10.1371/journal.pone.0012272. PLoS One. 2010. PMID: 20806064 Free PMC article.
References
-
- Black JA, Felts P, Smith KJ, Kocsis JD, Waxman SG. Distribution of sodium channels in chronically demyelinated spinal cord axons: immuno-ultrastructural localization and electrophysiological observations. Brain Res. 1991;544(1):59–70. - PubMed
-
- Blight AR. Miracles and molecules--progress in spinal cord repair. Nat Neurosci. 2002;5(Suppl):1051–4. - PubMed
-
- Blight AR, Tuszynski MH. Clinical trials in spinal cord injury. J Neurotrauma. 2006;23(3-4):586–93. - PubMed
-
- Bradbury EJ, McMahon SB. Spinal cord repair strategies: why do they work? Nat Rev Neurosci. 2006;7(8):644–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

