High-resolution electron microscopy of helical specimens: a fresh look at tobacco mosaic virus
- PMID: 17585939
- PMCID: PMC2025690
- DOI: 10.1016/j.jmb.2007.05.088
High-resolution electron microscopy of helical specimens: a fresh look at tobacco mosaic virus
Abstract
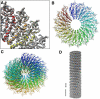
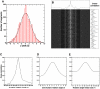
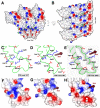
The treatment of helical objects as a string of single particles has become an established technique to resolve their three-dimensional (3D) structure using electron cryo-microscopy. It can be applied to a wide range of helical particles such as viruses, microtubules and helical filaments. We have made improvements to this approach using Tobacco Mosaic Virus (TMV) as a test specimen and obtained a map from 210,000 asymmetric units at a resolution better than 5 A. This was made possible by performing a full correction of the contrast transfer function of the microscope. Alignment of helical segments was helped by constraints derived from the helical symmetry of the virus. Furthermore, symmetrization was implemented by multiple inclusions of symmetry-related views in the 3D reconstruction. We used the density map to build an atomic model of TMV. The model was refined using a real-space refinement strategy that accommodates multiple conformers. The atomic model shows significant deviations from the deposited model for the helical form of TMV at the lower-radius region (residues 88 to 109). This region appears more ordered with well-defined secondary structure, compared with the earlier helical structure. The RNA phosphate backbone is sandwiched between two arginine side-chains, stabilizing the interaction between RNA and coat protein. A cluster of two or three carboxylates is buried in a hydrophobic environment isolating it from neighboring subunits. These carboxylates may represent the so-called Caspar carboxylates that form a metastable switch for viral disassembly. Overall, the observed differences suggest that the new model represents a different, more stable state of the virus, compared with the earlier published model.
Figures








Similar articles
-
Hydrogen-bonding networks and RNA bases revealed by cryo electron microscopy suggest a triggering mechanism for calcium switches.Proc Natl Acad Sci U S A. 2011 Jun 7;108(23):9637-42. doi: 10.1073/pnas.1018104108. Epub 2011 May 17. Proc Natl Acad Sci U S A. 2011. PMID: 21586634 Free PMC article.
-
4.6A Cryo-EM reconstruction of tobacco mosaic virus from images recorded at 300 keV on a 4k x 4k CCD camera.J Struct Biol. 2010 Sep;171(3):303-8. doi: 10.1016/j.jsb.2010.06.011. Epub 2010 Jun 15. J Struct Biol. 2010. PMID: 20558300 Free PMC article.
-
Elucidation of the viral disassembly switch of tobacco mosaic virus.EMBO Rep. 2019 Nov 5;20(11):e48451. doi: 10.15252/embr.201948451. Epub 2019 Sep 19. EMBO Rep. 2019. PMID: 31535454 Free PMC article.
-
Switching in the self-assembly of tobacco mosaic virus.Adv Biophys. 1990;26:157-85. doi: 10.1016/0065-227x(90)90011-h. Adv Biophys. 1990. PMID: 2082726 Review.
-
Cryo-EM Structure Determination Using Segmented Helical Image Reconstruction.Methods Enzymol. 2016;579:307-28. doi: 10.1016/bs.mie.2016.05.034. Epub 2016 Jun 28. Methods Enzymol. 2016. PMID: 27572732 Review.
Cited by
-
Probing bunyavirus N protein oligomerisation using mass spectrometry.Rapid Commun Mass Spectrom. 2014 Apr 15;28(7):793-800. doi: 10.1002/rcm.6841. Rapid Commun Mass Spectrom. 2014. PMID: 24573811 Free PMC article.
-
Force-producing ADP state of myosin bound to actin.Proc Natl Acad Sci U S A. 2016 Mar 29;113(13):E1844-52. doi: 10.1073/pnas.1516598113. Epub 2016 Mar 14. Proc Natl Acad Sci U S A. 2016. PMID: 26976594 Free PMC article.
-
Getting Hold of the Tobamovirus Particle-Why and How? Purification Routes over Time and a New Customizable Approach.Viruses. 2024 May 30;16(6):884. doi: 10.3390/v16060884. Viruses. 2024. PMID: 38932176 Free PMC article. Review.
-
A dose-rate effect in single-particle electron microscopy.J Struct Biol. 2008 Jan;161(1):92-100. doi: 10.1016/j.jsb.2007.09.017. Epub 2007 Oct 1. J Struct Biol. 2008. PMID: 17977018 Free PMC article.
-
Ring closure activates yeast γTuRC for species-specific microtubule nucleation.Nat Struct Mol Biol. 2015 Feb;22(2):132-7. doi: 10.1038/nsmb.2953. Epub 2015 Jan 19. Nat Struct Mol Biol. 2015. PMID: 25599398 Free PMC article.
References
-
- Henderson R. Realizing the potential of electron cryo-microscopy. Quarterly Reviews of Biophysics. 2004;37:3–13. - PubMed
-
- Henderson R, Baldwin JM, Ceska TA, Zemlin F, Beckmann E, Downing KH. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990;213:899–929. - PubMed
-
- De Rosier DJ, Klug A. Reconstruction of Three Dimensional Structures from Electron M icrographs. Nature. 1968;217:130–134. - PubMed
-
- Yonekura K, Maki-Yonekura S, Namba K. Complete atomic model of the bacterial flagellar filament by electron cryomicroscopy. Nature. 2003;424:643–50. - PubMed
-
- Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J Mol Biol. 2005;346:967–89. - PubMed
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

