Age-related changes in human macular Bruch's membrane as seen by quick-freeze/deep-etch
- PMID: 17586493
- PMCID: PMC2352152
- DOI: 10.1016/j.exer.2007.03.011
Age-related changes in human macular Bruch's membrane as seen by quick-freeze/deep-etch
Abstract
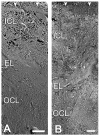
Lipid-containing inclusions have been observed in human Bruch's membrane (BrM) and are postulated to be associated with age-related maculopathy (ARM), a major cause of legal blindness in developed countries. The dehydration associated with specimen preparation for thin-section transmission electron microscopy causes loss of these inclusions. Better preservation of the ultrastructure of the inclusions and tissue is achieved by using a quick-freeze/deep-etch preparation. We use this technique to examine normal human macular BrM in order to characterize the deposition of the lipid-rich inclusions and their age-related accumulation within different layers of the tissue. We find that various inclusions mentioned in other studies can be formed by combinations of three basic structures: lipoprotein-like particles (LLPs), small granules (SGs) and membrane-like structures. These inclusions are associated with collagen and elastic fibrils by fine filaments. In younger eyes, these inclusions are found mostly in the elastic (EL) and outer collageneous layer (OCL) and occupy a small fraction of the interfibrillar spacing. As age increases, LLPs and SGs gradually fill the interfibrillar spacing of the EL and inner collageneous layer (ICL) of the tissue, and later form a new sublayer, the lipid wall, within the boundary region between the basal lamina of retinal pigment epithelium (RPE) and ICL. Because the formation of the lipid wall only occurs after these inclusions fill the ICL, and it seems unlikely that the LLPs can pass through the packed layer, this result suggests a possible RPE origin of the LLPs that make up the lipid wall.
Figures


















Similar articles
-
Morphometric analysis of lipoprotein-like particle accumulation in aging human macular Bruch's membrane.Invest Ophthalmol Vis Sci. 2008 Jun;49(6):2721-7. doi: 10.1167/iovs.07-1196. Epub 2008 Feb 22. Invest Ophthalmol Vis Sci. 2008. PMID: 18296655 Free PMC article.
-
Comparison of morphology of human macular and peripheral Bruch's membrane in older eyes.Curr Eye Res. 2007 Sep;32(9):791-9. doi: 10.1080/02713680701550660. Curr Eye Res. 2007. PMID: 17882712 Free PMC article.
-
Quick-freeze/deep-etch visualization of age-related lipid accumulation in Bruch's membrane.Invest Ophthalmol Vis Sci. 2003 Apr;44(4):1753-9. doi: 10.1167/iovs.02-0496. Invest Ophthalmol Vis Sci. 2003. PMID: 12657618
-
Aging, age-related macular degeneration, and the response-to-retention of apolipoprotein B-containing lipoproteins.Prog Retin Eye Res. 2009 Nov;28(6):393-422. doi: 10.1016/j.preteyeres.2009.08.001. Epub 2009 Aug 19. Prog Retin Eye Res. 2009. PMID: 19698799 Free PMC article. Review.
-
[Drusen in Bruch's membrane. Their significance for the pathogenesis and therapy of age-associated macular degeneration].Ophthalmologe. 1992 Oct;89(5):363-86. Ophthalmologe. 1992. PMID: 1304217 Review. German.
Cited by
-
HISTOLOGY OF GEOGRAPHIC ATROPHY SECONDARY TO AGE-RELATED MACULAR DEGENERATION: A Multilayer Approach.Retina. 2018 Oct;38(10):1937-1953. doi: 10.1097/IAE.0000000000002182. Retina. 2018. PMID: 29746415 Free PMC article.
-
Watching the human retina breath in real time and the slowing of mitochondrial respiration with age.Sci Rep. 2023 Apr 20;13(1):6445. doi: 10.1038/s41598-023-32897-7. Sci Rep. 2023. PMID: 37081065 Free PMC article.
-
Complementing apolipoprotein secretion by cultured retinal pigment epithelium.Proc Natl Acad Sci U S A. 2011 Nov 15;108(46):18569-70. doi: 10.1073/pnas.1115497108. Epub 2011 Nov 7. Proc Natl Acad Sci U S A. 2011. PMID: 22065764 Free PMC article. No abstract available.
-
Apolipoprotein B-containing lipoproteins in retinal aging and age-related macular degeneration.J Lipid Res. 2010 Mar;51(3):451-67. doi: 10.1194/jlr.R002238. Epub 2009 Sep 29. J Lipid Res. 2010. PMID: 19797256 Free PMC article. Review.
-
Passage of low-density lipoproteins through Bruch's membrane and choroid.Exp Eye Res. 2011 Dec;93(6):947-55. doi: 10.1016/j.exer.2011.10.016. Epub 2011 Nov 3. Exp Eye Res. 2011. PMID: 22063729 Free PMC article.
References
-
- Anderson DH, Fisher SK, Steinberg RH. Mammalian cones: disc shedding, phagocytosis, and renewal. Invest Ophthalmol Vis Sci. 1978;17:117–133. - PubMed
-
- Bird AC, Marshall J. Retinal pigment epithelial detachments in the elderly. Trans Ophthalmol Soc U K. 1986;105:674–682. - PubMed
-
- Bobryshev YV, Lord RS. Accumulation of co-localised unesterified cholesterol and neutral lipids within vacuolised elastin fibres in athero-prone areas of the human aorta. Atherosclerosis. 1999;142:121–131. - PubMed
-
- Bocan TM, Brown SA, Guyton JR. Human aortic fibrolipid lesions. Immunochemical localization of apolipoprotein B and apolipoprotein A. Arteriosclerosis. 1988;8:499–508. - PubMed
-
- Chang MY, Potter-Perigo S, Wight TN, Chait A. Oxidized LDL bind to nonproteoglycan components of smooth muscle extracellular matrices. J Lipid Res. 2001;42:824–833. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

