A novel exclusion mechanism for carbon resource partitioning in Aggregatibacter actinomycetemcomitans
- PMID: 17586632
- PMCID: PMC1951915
- DOI: 10.1128/JB.00554-07
A novel exclusion mechanism for carbon resource partitioning in Aggregatibacter actinomycetemcomitans
Abstract
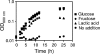
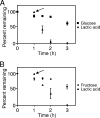
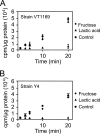
The bacterium Aggregatibacter actinomycetemcomitans is a common commensal of the human oral cavity and the putative causative agent of the disease localized aggressive periodontitis. A. actinomycetemcomitans is a slow-growing bacterium that possesses limited metabolic machinery for carbon utilization. This likely impacts its ability to colonize the oral cavity, where growth and community composition is mediated by carbon availability. We present evidence that in the presence of the in vivo relevant carbon substrates glucose, fructose, and lactate A. actinomycetemcomitans preferentially metabolizes lactate. This preference for lactate exists despite the fact that A. actinomycetemcomitans grows faster and obtains higher cell yields during growth with carbohydrates. The preference for lactate is mediated by a novel exclusion mechanism in which metabolism of lactate inhibits carbohydrate uptake. Coculture studies reveal that A. actinomycetemcomitans utilizes lactate produced by the oral bacterium Streptococcus gordonii, suggesting the potential for cross-feeding in the oral cavity.
Figures






References
-
- Anthon, G. E., and D. M. Barrett. 2003. Modified method for the determination of pyruvic acid with dinitrophenylhydrazine in the assessment of onion pungency. J. Sci. Food Agric. 83:1210-1213.
-
- Ausubel, F., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1997. Short protocols in molecular biology, 3rd ed. John Wiley & Sons, Inc., New York, NY.
-
- Bergey, N., R. Krieg, and J. G. Holt. 1984. Bergey's manual of systematic bacteriology, vol. 4, p. 537. The Williams & Wilkins Co., Baltimore, MD.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

