SadC reciprocally influences biofilm formation and swarming motility via modulation of exopolysaccharide production and flagellar function
- PMID: 17586642
- PMCID: PMC2168701
- DOI: 10.1128/JB.00585-07
SadC reciprocally influences biofilm formation and swarming motility via modulation of exopolysaccharide production and flagellar function
Abstract
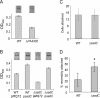
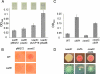
Pseudomonas aeruginosa has served as an important organism in the study of biofilm formation; however, we still lack an understanding of the mechanisms by which this microbe transitions to a surface lifestyle. A recent study of the early stages of biofilm formation implicated the control of flagellar reversals and production of an exopolysaccharide (EPS) as factors in the establishment of a stable association with the substratum and swarming motility. Here we present evidence that SadC (PA4332), an inner membrane-localized diguanylate cyclase, plays a role in controlling these cellular functions. Deletion of the sadC gene results in a strain that is defective in biofilm formation and a hyperswarmer, while multicopy expression of this gene promotes sessility. A DeltasadC mutant was additionally found to be deficient in EPS production and display altered reversal behavior while swimming in high-viscosity medium, two behaviors proposed to influence biofilm formation and swarming motility. Epistasis analysis suggests that the sadC gene is part of a genetic pathway that allows for the concomitant regulation of these aspects of P. aeruginosa surface behavior. We propose that SadC and the phosphodiesterase BifA (S. L. Kuchma et al., J. Bacteriol. 189:8165-8178, 2007), via modulating levels of the signaling molecule cyclic-di-GMP, coregulate swarming motility and biofilm formation as P. aeruginosa transitions from a planktonic to a surface-associated lifestyle.
Figures





References
-
- Branda, S. S., A. Vik, L. Friedman, and R. Kolter. 2005. Biofilms: the matrix revisited. Trends Microbiol. 13:20-26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

