Metabolic engineering of Bacillus subtilis for ethanol production: lactate dehydrogenase plays a key role in fermentative metabolism
- PMID: 17586670
- PMCID: PMC1950962
- DOI: 10.1128/AEM.00625-07
Metabolic engineering of Bacillus subtilis for ethanol production: lactate dehydrogenase plays a key role in fermentative metabolism
Abstract
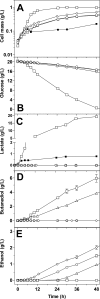
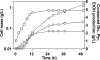
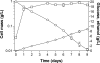
Wild-type Bacillus subtilis ferments 20 g/liter glucose in 48 h, producing lactate and butanediol, but not ethanol or acetate. To construct an ethanologenic B. subtilis strain, homologous recombination was used to disrupt the native lactate dehydrogenase (LDH) gene (ldh) by chromosomal insertion of the Zymomonas mobilis pyruvate decarboxylase gene (pdc) and alcohol dehydrogenase II gene (adhB) under the control of the ldh native promoter. The values of the intracellular PDC and ADHII enzymatic activities of the engineered B. subtilis BS35 strain were similar to those found in an ethanologenic Escherichia coli strain. BS35 produced ethanol and butanediol; however, the cell growth and glucose consumption rates were reduced by 70 and 65%, respectively, in comparison to those in the progenitor strain. To eliminate butanediol production, the acetolactate synthase gene (alsS) was inactivated. In the BS36 strain (BS35 delta alsS), ethanol production was enhanced, with a high yield (89% of the theoretical); however, the cell growth and glucose consumption rates remained low. Interestingly, kinetic characterization of LDH from B. subtilis showed that it is able to oxidize NADH and NADPH. The expression of the transhydrogenase encoded by udhA from E. coli allowed a partial recovery of the cell growth rate and an early onset of ethanol production. Beyond pyruvate-to-lactate conversion and NADH oxidation, an additional key physiological role of LDH for glucose consumption under fermentative conditions is suggested. Long-term cultivation showed that 8.9 g/liter of ethanol can be obtained using strain BS37 (BS35 delta alsS udhA+). As far as we know, this is the highest ethanol titer and yield reported with a B. subtilis strain.
Figures

Similar articles
-
NADH plays the vital role for chiral pure D-(-)-2,3-butanediol production in Bacillus subtilis under limited oxygen conditions.Biotechnol Bioeng. 2014 Oct;111(10):2126-31. doi: 10.1002/bit.25265. Epub 2014 May 28. Biotechnol Bioeng. 2014. PMID: 24788512
-
Regulation of the NADH pool and NADH/NADPH ratio redistributes acetoin and 2,3-butanediol proportion in Bacillus subtilis.Biotechnol J. 2015 Aug;10(8):1298-306. doi: 10.1002/biot.201400577. Biotechnol J. 2015. PMID: 26129872
-
Fermentative metabolism of Bacillus subtilis: physiology and regulation of gene expression.J Bacteriol. 2000 Jun;182(11):3072-80. doi: 10.1128/JB.182.11.3072-3080.2000. J Bacteriol. 2000. PMID: 10809684 Free PMC article.
-
Ethanol production by engineered thermophiles.Curr Opin Biotechnol. 2015 Jun;33:130-41. doi: 10.1016/j.copbio.2015.02.006. Epub 2015 Mar 5. Curr Opin Biotechnol. 2015. PMID: 25745810 Review.
-
Microbial Routes to (2R,3R)-2,3-Butanediol: Recent Advances and Future Prospects.Curr Top Med Chem. 2017;17(21):2433-2439. doi: 10.2174/1568026617666170504101646. Curr Top Med Chem. 2017. PMID: 28474550 Review.
Cited by
-
Transcriptomic and metabolomic profiling of Zymomonas mobilis during aerobic and anaerobic fermentations.BMC Genomics. 2009 Jan 20;10:34. doi: 10.1186/1471-2164-10-34. BMC Genomics. 2009. PMID: 19154596 Free PMC article.
-
Progress in research and application development of surface display technology using Bacillus subtilis spores.Appl Microbiol Biotechnol. 2020 Mar;104(6):2319-2331. doi: 10.1007/s00253-020-10348-x. Epub 2020 Jan 27. Appl Microbiol Biotechnol. 2020. PMID: 31989224 Free PMC article. Review.
-
Genomic insights and functional evaluation of Lacticaseibacillus paracasei EG005: a promising probiotic with enhanced antioxidant activity.Front Microbiol. 2024 Oct 14;15:1477152. doi: 10.3389/fmicb.2024.1477152. eCollection 2024. Front Microbiol. 2024. PMID: 39469458 Free PMC article.
-
Recent advances in engineering the central carbon metabolism of industrially important bacteria.Microb Cell Fact. 2012 Apr 30;11:50. doi: 10.1186/1475-2859-11-50. Microb Cell Fact. 2012. PMID: 22545791 Free PMC article. Review.
-
Moonlighting role of a poly-gamma-glutamate synthetase component from Bacillus subtilis: insight into novel extrachromosomal DNA maintenance.Appl Environ Microbiol. 2011 Apr;77(8):2796-8. doi: 10.1128/AEM.02649-10. Epub 2011 Feb 25. Appl Environ Microbiol. 2011. PMID: 21357437 Free PMC article.
References
-
- Aarnikunnas, J., N. von Weymarn, K. Rönnholm, M. Leisola, and A. Palva. 2003. Metabolic engineering of Lactobacillus fermentum for production of mannitol and pure l-lactic acid or pyruvate. Biotechnol. Bioeng. 82:653-663. - PubMed
-
- Barbosa, M. F. S., and L. O. Ingram. 1994. Expression of the Zymomonas mobilis alcohol dehydrogenase II (adhB) and pyruvate decarboxylase (pdc) genes in Bacillus. Curr. Microbiol. 28:279-282.
-
- Beall, D. S., K. Ohta, and L. O. Ingram. 1991. Parametric studies of ethanol production from xylose and other sugars by recombinant Escherichia coli. Biotechnol. Bioeng. 38:296-303. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

