Rab27b regulates mast cell granule dynamics and secretion
- PMID: 17587407
- PMCID: PMC2063611
- DOI: 10.1111/j.1600-0854.2007.00571.x
Rab27b regulates mast cell granule dynamics and secretion
Abstract
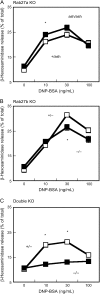
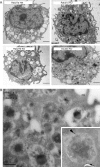
The Rab GTPase family regulates membrane domain organization and vesicular transport pathways. Recent studies indicate that one member of the family, Rab27a, regulates transport of lysosome-related organelles in specialized cells, such as melanosomes and lytic granules. Very little is known about the related isoform, Rab27b. Here we used genetically modified mice to study the involvement of the Rab27 proteins in mast cells, which play key roles in allergic responses. Both Rab27a and Rab27b isoforms are expressed in bone marrow-derived mast cells (BMMC) and localize to secretory granules. Nevertheless, secretory defects as measured by beta-hexosaminidase release in vitro and passive cutaneous anaphylaxis in vivo were found only in Rab27b and double Rab27 knockout (KO) mice. Immunofluorescence studies suggest that a subset of Rab27b and double Rab27-deficient BMMCs exhibit mild clustering of granules. Quantitative analysis of live-cell time-lapse imaging revealed that BMMCs derived from double Rab27 KO mice showed almost 10-fold increase in granules exhibiting fast movement (>1.5 microm/s), which could be disrupted by nocodazole. These results suggest that Rab27 proteins, particularly Rab27b, play a crucial role in mast cell degranulation and that their action regulates the transition from microtubule to actin-based motility.
Figures




References
-
- Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. - PubMed
-
- Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218–230. - PubMed
-
- Siraganian RP. Mast cell signal transduction from the high-affinity IgE receptor. Curr Opin Immunol. 2003;15:639–646. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

