CVAK104 is a novel regulator of clathrin-mediated SNARE sorting
- PMID: 17587408
- PMCID: PMC2239300
- DOI: 10.1111/j.1600-0854.2007.00576.x
CVAK104 is a novel regulator of clathrin-mediated SNARE sorting
Abstract
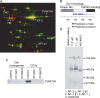
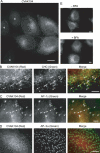
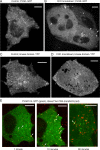
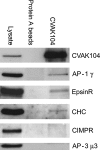
Clathrin-coated vesicles (CCVs) mediate transport between the plasma membrane, endosomes and the trans Golgi network. Using comparative proteomics, we have identified coated-vesicle-associated kinase of 104 kDa (CVAK104) as a candidate accessory protein for CCV-mediated trafficking. Here, we demonstrate that the protein colocalizes with clathrin and adaptor protein-1 (AP-1), and that it is associated with a transferrin-positive endosomal compartment. Consistent with these observations, clathrin as well as the cargo adaptors AP-1 and epsinR can be coimmunoprecipitated with CVAK104. Small interfering RNA (siRNA) knockdown of CVAK104 in HeLa cells results in selective loss of the SNARE proteins syntaxin 8 and vti1b from CCVs. Morpholino-mediated knockdown of CVAK104 in Xenopus tropicalis causes severe developmental defects, including a bent body axis and ventral oedema. Thus, CVAK104 is an evolutionarily conserved protein involved in SNARE sorting that is essential for normal embryonic development.
Figures


References
-
- Robinson MS. Adaptable adaptors for coated vesicles. Trends Cell Biol. 2004;14:167–174. - PubMed
-
- Edeling MA. Life of a clathrin coat: insights from clathrin and AP structures. Nat Rev Mol Cell Biol. 2006;7:32–44. - PubMed
-
- Traub LM. Common principles in clathrin-mediated sorting at the Golgi and the plasma membrane. Biochim Biophys Acta. 2005;1744:415–437. - PubMed
-
- Bonifacino JS. Retrograde transport from endosomes to the trans-Golgi network. Nat Rev Mol Cell Biol. 2006;7:568–579. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

