Folding versus aggregation: polypeptide conformations on competing pathways
- PMID: 17588526
- PMCID: PMC2706318
- DOI: 10.1016/j.abb.2007.05.015
Folding versus aggregation: polypeptide conformations on competing pathways
Abstract
Protein aggregation has now become recognised as an important and generic aspect of protein energy landscapes. Since the discovery that numerous human diseases are caused by protein aggregation, the biophysical characterisation of misfolded states and their aggregation mechanisms has received increased attention. Utilising experimental techniques and computational approaches established for the analysis of protein folding reactions has ensured rapid advances in the study of pathways leading to amyloid fibrils and amyloid-related aggregates. Here we describe recent experimental and theoretical advances in the elucidation of the conformational properties of dynamic, heterogeneous and/or insoluble protein ensembles populated on complex, multidimensional protein energy landscapes. We discuss current understanding of aggregation mechanisms in this context and describe how the synergy between biochemical, biophysical and cell-biological experiments are beginning to provide detailed insights into the partitioning of non-native species between protein folding and aggregation pathways.
Figures

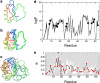



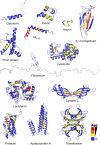
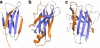
References
-
- Levinthal C. J. Chim. Phys. 1968;65:44–45.
-
- Fersht A.R., Daggett V. Cell. 2002;108:573–582. - PubMed
-
- Dinner A.R., Sali A., Smith L.J., Dobson C.M., Karplus M. Trends Biochem. Sci. 2000;25:331–339. - PubMed
-
- Wolynes P.G., Onuchic J.N., Thirumalai D. Science. 1995;267:1619–1620. - PubMed
-
- Dill K.A., Chan H.S. Nature Struct. Biol. 1997;4:10–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

