Heart valve function: a biomechanical perspective
- PMID: 17588873
- PMCID: PMC2440402
- DOI: 10.1098/rstb.2007.2122
Heart valve function: a biomechanical perspective
Erratum in
- Philos Trans R Soc Lond B Biol Sci. 2008 Jul 27;363(1502):2471
Abstract
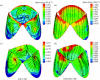
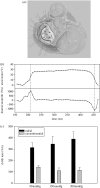
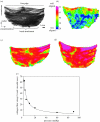
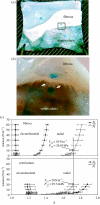
Heart valves (HVs) are cardiac structures whose physiological function is to ensure directed blood flow through the heart over the cardiac cycle. While primarily passive structures that are driven by forces exerted by the surrounding blood and heart, this description does not adequately describe their elegant and complex biomechanical function. Moreover, they must replicate their cyclic function over an entire lifetime, with an estimated total functional demand of least 3x10(9) cycles. As in many physiological systems, one can approach HV biomechanics from a multi-length-scale approach, since mechanical stimuli occur and have biological impact at the organ, tissue and cellular scales. The present review focuses on the functional biomechanics of HVs. Specifically, we refer to the unique aspects of valvular function, and how the mechanical and mechanobiological behaviours of the constituent biological materials (e.g. extracellular matrix proteins and cells) achieve this remarkable feat. While we focus on the work from the authors' respective laboratories, the works of most investigators known to the authors have been included whenever appropriate. We conclude with a summary and underscore important future trends.
Figures







References
-
- Adamczyk M.M, Vesely I. Characteristics of compressive strains in porcine aortic valves cusps. J. Heart Valve Dis. 2002;11:75–83. - PubMed
-
- Akdemir R, Ozhan H, Bulur S, Unlu H, Gunduz H, Arinc H, Yildiz A, Uyan C. Color M-mode regurgitant flow propagation velocity: a new echocardiographic method for grading of mitral regurgitation. Echocardiography. 2005;22:713–722. doi:10.1111/j.1540-8175.2005.00101.x - DOI - PubMed
-
- Arts T, Meerbaum S, Reneman R, Corday E. Stresses in the closed mitral valve: a model study. J. Biomech. 1983;16:539–547. doi:10.1016/0021-9290(83)90068-4 - DOI - PubMed
-
- Bairati A, DeBiasi S. Presence of a smooth muscle system in aortic valve leaflets. Anat. Embryol. 1981;161:329–340. doi:10.1007/BF00301830 - DOI - PubMed
-
- Bellhouse B.J, Bellhouse F.H. Fluid mechanics of the mitral valve. Nature. 1969;224:615–618. doi:10.1038/224615a0 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
