Markers and methods for cell sorting of human embryonic stem cell-derived neural cell populations
- PMID: 17588935
- PMCID: PMC2238728
- DOI: 10.1634/stemcells.2006-0744
Markers and methods for cell sorting of human embryonic stem cell-derived neural cell populations
Abstract
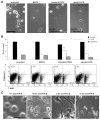
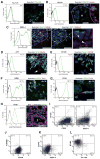
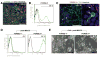
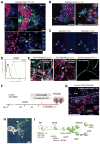
Neural cells differentiated in vitro from human embryonic stem cells (hESC) exhibit broad cellular heterogeneity with respect to developmental stage and lineage specification. Here, we describe standard conditions for the use and discovery of markers for analysis and cell selection of hESC undergoing neuronal differentiation. To generate better-defined cell populations, we established a working protocol for sorting heterogeneous hESC-derived neural cell populations by fluorescence-activated cell sorting (FACS). Using genetically labeled synapsin-green fluorescent protein-positive hESC-derived neurons as a proof of principle, we enriched viable differentiated neurons by FACS. Cell sorting methodology using surface markers was developed, and a comprehensive profiling of surface antigens was obtained for immature embryonic stem cell types (such as stage-specific embryonic antigen [SSEA]-3, -4, TRA-1-81, TRA-1-60), neural stem and precursor cells (such as CD133, SSEA-1 [CD15], A2B5, forebrain surface embryonic antigen-1, CD29, CD146, p75 [CD271]), and differentiated neurons (such as CD24 or neural cell adhesion molecule [NCAM; CD56]). At later stages of neural differentiation, NCAM (CD56) was used to isolate hESC-derived neurons by FACS. Such FACS-sorted hESC-derived neurons survived in vivo after transplantation into rodent brain. These results and concepts provide (a) a feasible approach for experimental cell sorting of differentiated neurons, (b) an initial survey of surface antigens present during neural differentiation of hESC, and (c) a framework for developing cell selection strategies for neural cell-based therapies.
Conflict of interest statement
Disclosure of potential conflicts of interest
The authors indicate no potential conflicts of interest.
Figures


References
-
- Stewart MH, Bosse M, Chadwick K, et al. Clonal isolation of hESCs reveals heterogeneity within the pluripotent stem cell compartment. Nat Methods. 2006;3:807–815. - PubMed
-
- Isacson O, Bjorklund LM, Schumacher JM. Toward full restoration of synaptic and terminal function of the dopaminergic system in Parkinson’s disease by stem cells. Ann Neurol. 2003;53(suppl 3):S135–S146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

