Interaction of cartilage oligomeric matrix protein/thrombospondin 5 with aggrecan
- PMID: 17588949
- PMCID: PMC2905148
- DOI: 10.1074/jbc.M611390200
Interaction of cartilage oligomeric matrix protein/thrombospondin 5 with aggrecan
Abstract
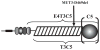
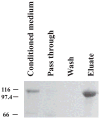
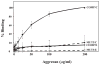
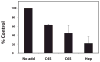
Cartilage oligomeric matrix protein/thrombospondin 5 (COMP/TSP5) is a major component of the extracellular matrix (ECM) of the musculoskeletal system. Its importance is underscored by its association with several growth disorders. In this report, we investigated its interaction with aggrecan, a major component of cartilage ECM. We also tested a COMP/TSP5 mutant, designated MUT3 that accounts for 30% of human pseudoachondroplasia cases, to determine if the mutation affects function. Using a solid-phase binding assay, we have shown that COMP/TSP5 can bind aggrecan. This binding was decreased with MUT3, or when COMP/TSP5 was treated with EDTA, indicating the presence of a conformation-dependent aggrecan binding site. Soluble glycosaminoglycans (GAGs) partially inhibited binding, suggesting that the interaction was mediated in part through aggrecan GAG side chains. Using affinity co-electrophoresis, we showed that COMP/TSP5, in its calcium-replete conformation, bound to heparin, chondroitin sulfates, and heparan sulfate; this binding was reduced with EDTA treatment of COMP/TSP5. MUT3 showed weaker binding than calcium-repleted COMP/TSP5. Using recombinant COMP/TSP5 fragments, we found that the "signature domain" could bind to aggrecan, suggesting that this domain can mediate the interaction of COMP/TSP5 and aggrecan. In summary, our data indicate that COMP/TSP5 is an aggrecan-binding protein, and this interaction is regulated by the calcium-sensitive conformation of COMP/TSP5; interaction of COMP with aggrecan can be mediated through the GAG side chains on aggrecan and the "signature domain" of COMP/TSP5. Our results suggest that COMP/TSP5 may function to support matrix interactions in cartilage ECM.
Figures


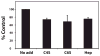
References
-
- Oldberg A, Antonsson P, Lindblom K, Heinegard D. J Biol Chem. 1992;267:22346–22350. - PubMed
-
- Newton G, Weremowicz S, Morton CC, Copeland NG, Gilbert DJ, Jenkins NA, Lawler J. Genomics. 1994;24:435–439. - PubMed
-
- Di Cesare PE, Fang C, Leslie MP, Tulli H, Perris R, Carlson CS. J Orthop Res. 2000;18:713–720. - PubMed
-
- DiCesare PE, Carlson CS, Stollerman ES, Chen FS, Leslie M, Perris R. FEBS Lett. 1997;412:249–252. - PubMed
-
- Di Cesare PE, Hauser N, Lehman D, Pasumarti S, Paulsson M. FEBS Lett. 1994;354:237–240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

