Relationship of treatment delays and mortality in patients undergoing fibrinolysis and primary percutaneous coronary intervention. The Global Registry of Acute Coronary Events
- PMID: 17591643
- PMCID: PMC2095752
- DOI: 10.1136/hrt.2006.112847
Relationship of treatment delays and mortality in patients undergoing fibrinolysis and primary percutaneous coronary intervention. The Global Registry of Acute Coronary Events
Abstract
Objective: Treatment delays may result in different clinical outcomes in patients with ST-segment elevation myocardial infarction (STEMI) who receive fibrinolytic therapy vs primary percutaneous coronary intervention (PCI). The aim of this analysis was to examine how treatment delays relate to 6-month mortality in reperfusion-treated patients enrolled in the Global Registry of Acute Coronary Events (GRACE).
Design: Prospective, observational cohort study.
Setting: 106 hospitals in 14 countries.
Patients: 3959 patients who presented with STEMI within 6 h of symptom onset and received reperfusion with either a fibrin-specific fibrinolytic drug or primary PCI.
Main outcome measures: 6-month mortality.
Methods: Multivariable logistic regression was used to assess the relationship between outcomes and treatment delay separately in each cohort, with time modelled with a quadratic term after adjusting for covariates from the GRACE risk score.
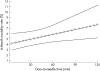
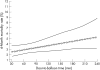
Results: A total of 1786 (45.1%) patients received fibrinolytic therapy, and 2173 (54.9%) underwent primary PCI. After multivariable adjustment, longer treatment delays were associated with a higher 6-month mortality in both fibrinolytic therapy and primary PCI patients (p<0.001 for both cohorts). For patients who received fibrinolytic therapy, 6-month mortality increased by 0.30% per 10-min delay in door-to-needle time between 30 and 60 min compared with 0.18% per 10-min delay in door-to-balloon time between 90 and 150 min for patients undergoing primary PCI.
Conclusions: Treatment delays in reperfusion therapy are associated with higher 6-month mortality, but this relationship may be even more critical in patients receiving fibrinolytic therapy.
Conflict of interest statement
Competing interests: B K Nallamothu: none. K A A Fox: British Heart foundation, Medical Research Council, The Wellcome Trust, Aventis, Sanofi Synthelabo, GlaxoSmithKline, Bristol‐Myers Squibb. B M Kennelly: sanofi‐aventis. F Van de Werf: sanofi‐aventis. J M Gore: sanofi‐aventis. P G Steg: Speakers bureau; Boehringer‐Ingelheim, BMS, GSK, MSD, Novartis, Nycomed, sanofi‐aventis, Sankyo, Servier, ZLB‐Behring. Consulting/advisory board: AstraZeneca, BMS, GSK, MSD, Pfizer, sanofi‐aventis, Servier, Takeda. Stockholding: none. C B Granger: sanofi‐aventis, Procter and Gamble, Alexion, Bristol‐Myers Squibb, Boehringer Ingelheim, GlaxoSmithKline, Novartis, The Medicines Company. O H Dabbous: none. E Kline‐Rogers: none. K A Eagle: Biosite, Bristol Myers Squibb, Cardiac Sciences, Blue Cross Blue Shield of Michigan, Hewlett Foundation, Mardigian Fund, Pfizer, sanofi‐aventis, Varbedian fund, National Heart, Lung & Blood NIH.
References
-
- Giugliano R P, Braunwald E. Selecting the best reperfusion strategy in ST‐elevation myocardial infarction: it's all a matter of time. Circulation 20031082828–2830. - PubMed
-
- Betriu A, Masotti M. Comparison of mortality rates in acute myocardial infarction treated by percutaneous coronary intervention versus fibrinolysis. Am J Cardiol 200595100–101. - PubMed
-
- Boersma E. Does time matter? A pooled analysis of randomized clinical trials comparing primary percutaneous coronary intervention and in‐hospital fibrinolysis in acute myocardial infarction patients. Eur Heart J 200627779–788. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous