WAVE2 regulates high-affinity integrin binding by recruiting vinculin and talin to the immunological synapse
- PMID: 17591693
- PMCID: PMC1952166
- DOI: 10.1128/MCB.00136-07
WAVE2 regulates high-affinity integrin binding by recruiting vinculin and talin to the immunological synapse
Abstract
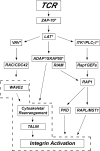
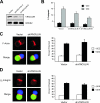
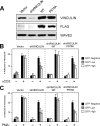
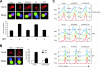
T-cell-receptor (TCR)-mediated integrin activation is required for T-cell-antigen-presenting cell conjugation and adhesion to extracellular matrix components. While it has been demonstrated that the actin cytoskeleton and its regulators play an essential role in this process, no mechanism has been established which directly links TCR-induced actin polymerization to the activation of integrins. Here, we demonstrate that TCR stimulation results in WAVE2-ARP2/3-dependent F-actin nucleation and the formation of a complex containing WAVE2, ARP2/3, vinculin, and talin. The verprolin-connecting-acidic (VCA) domain of WAVE2 mediates the formation of the ARP2/3-vinculin-talin signaling complex and talin recruitment to the immunological synapse (IS). Interestingly, although vinculin is not required for F-actin or integrin accumulation at the IS, it is required for the recruitment of talin. In addition, RNA interference of either WAVE2 or vinculin inhibits activation-dependent induction of high-affinity integrin binding to VCAM-1. Overall, these findings demonstrate a mechanism in which signals from the TCR produce WAVE2-ARP2/3-mediated de novo actin polymerization, leading to integrin clustering and high-affinity binding through the recruitment of vinculin and talin.
Figures
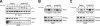




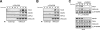
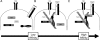
References
-
- Calderwood, D. A., R. Zent, R. Grant, D. J. Rees, R. O. Hynes, and M. H. Ginsberg. 1999. The Talin head domain binds to integrin beta subunit cytoplasmic tails and regulates integrin activation. J. Biol. Chem. 274:28071-28074. - PubMed
-
- Campbell, I. D., and M. H. Ginsberg. 2004. The talin-tail interaction places integrin activation on FERM ground. Trends Biochem. Sci. 29:429-435. - PubMed
-
- Cannon, J. L., and J. K. Burkhardt. 2004. Differential roles for Wiskott-Aldrich syndrome protein in immune synapse formation and IL-2 production. J. Immunol. 173:1658-1662. - PubMed
-
- Cannon, J. L., C. M. Labno, G. Bosco, A. Seth, M. H. McGavin, K. A. Siminovitch, M. K. Rosen, and J. K. Burkhardt. 2001. Wasp recruitment to the T cell:APC contact site occurs independently of Cdc42 activation. Immunity 15:249-259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-AI065474/AI/NIAID NIH HHS/United States
- R01-AI038474/AI/NIAID NIH HHS/United States
- R01 AI065474/AI/NIAID NIH HHS/United States
- T32-AI07425/AI/NIAID NIH HHS/United States
- T32-CA009138/CA/NCI NIH HHS/United States
- T32 CA009138/CA/NCI NIH HHS/United States
- R01-AI031126/AI/NIAID NIH HHS/United States
- R01-AI060921/AI/NIAID NIH HHS/United States
- T32 AI007425/AI/NIAID NIH HHS/United States
- R01 AI031126/AI/NIAID NIH HHS/United States
- R01 AI060921/AI/NIAID NIH HHS/United States
- R01 AI038474/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous
