Exosomes released from infected macrophages contain Mycobacterium avium glycopeptidolipids and are proinflammatory
- PMID: 17591775
- PMCID: PMC3636815
- DOI: 10.1074/jbc.M702277200
Exosomes released from infected macrophages contain Mycobacterium avium glycopeptidolipids and are proinflammatory
Abstract
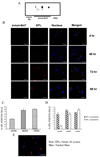
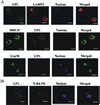
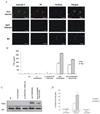
Mycobacterium avium is a major opportunistic pathogen in HIV-positive individuals and is responsible for increased morbidity and mortality in AIDS patients. M. avium express glycopeptidolipids (GPLs) as a major cell wall constituent, and recent studies suggest that GPLs play an important role in M. avium pathogenesis. In the present study we show that M. avium-infected macrophages release GPLs, which are trafficked from the phagosome through the endocytic network to multivesicular bodies. Prior studies have shown that multivesicular bodies can fuse with the plasma membrane releasing small 50 to 100 nm vesicles known as exosomes. We found that M. avium-infected macrophages release exosomes containing GPLs leading to the transfer of GPLs from infected to uninfected macrophages. Interestingly, exosomes isolated from M. avium-infected but not from uninfected macrophages can stimulate a proinflammatory response in resting macrophages. This proinflammatory response is dependent on Toll like receptor (TLR) 2, TLR4, and MyD88 suggesting that released exosomes contain M. avium-expressed TLR ligands. Our studies are the first to demonstrate that exosomes isolated from mycobacteria-infected macrophages can induce a proinflammatory response, and we hypothesize that exosomes play an important role in immune surveillance during intracellular bacteria infections.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

