Pneumococcal cell wall-induced meningitis impairs adult hippocampal neurogenesis
- PMID: 17591796
- PMCID: PMC1951165
- DOI: 10.1128/IAI.01679-06
Pneumococcal cell wall-induced meningitis impairs adult hippocampal neurogenesis
Abstract
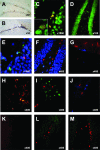
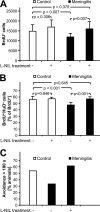
Bacterial meningitis is a major infectious cause of neuronal degeneration in the hippocampus. Neurogenesis, a continuous process in the adult hippocampus, could ameliorate such loss. Yet the high rate of sequelae from meningitis suggests that this repair mechanism is inefficient. Here we used a mouse model of nonreplicative bacterial meningitis to determine the impact of transient intracranial inflammation on adult neurogenesis. Experimental meningitis resulted in a net loss of neurons, diminished volume, and impaired neurogenesis in the dentate gyrus for weeks following recovery from the insult. Inducible nitric oxide synthase (iNOS) immunoreactivity was prominent in microglia in nonproliferating areas of the dentate gyrus and hilus region after meningitis induction. Treatment with the specific iNOS inhibitor N6-(1-iminoethyl)-L-lysine restored neurogenesis in experimental meningitis. These data suggest that local central nervous system inflammation in and of itself suppresses adult neurogenesis by affecting both proliferation and neuronal differentiation. Repair of cognitive dysfunction following meningitis could be improved by intervention to interrupt these actively suppressive effects.
Figures


Similar articles
-
Enriched environment fails to increase meningitis-induced neurogenesis and spatial memory in a mouse model of pneumococcal meningitis.J Neurosci Res. 2009 Jun;87(8):1877-83. doi: 10.1002/jnr.22010. J Neurosci Res. 2009. PMID: 19170185
-
Inducible nitric oxide synthase mediates hippocampal caspase-3 activation in pneumococcal meningitis.Int J Neurosci. 2009;119(4):455-9. doi: 10.1080/00207450802479970. Int J Neurosci. 2009. PMID: 19229714
-
Pneumococcal meningitis induces apoptosis in recently postmitotic immature neurons in the dentate gyrus of neonatal rats.Dev Neurosci. 2007;29(1-2):134-42. doi: 10.1159/000096218. Dev Neurosci. 2007. PMID: 17148956
-
Experimental studies of pneumococcal meningitis.Dan Med Bull. 2010 Jan;57(1):B4119. Dan Med Bull. 2010. PMID: 20175949 Review.
-
Comparative neuroscience of stimulant-induced memory dysfunction: role for neurogenesis in the adult hippocampus.Behav Pharmacol. 2010 Sep;21(5-6):379-93. doi: 10.1097/FBP.0b013e32833e16b6. Behav Pharmacol. 2010. PMID: 20700045 Review.
Cited by
-
Microglia development and function.Annu Rev Immunol. 2014;32:367-402. doi: 10.1146/annurev-immunol-032713-120240. Epub 2014 Jan 22. Annu Rev Immunol. 2014. PMID: 24471431 Free PMC article. Review.
-
Pre-infection physical exercise decreases mortality and stimulates neurogenesis in bacterial meningitis.J Neuroinflammation. 2012 Jul 10;9:168. doi: 10.1186/1742-2094-9-168. J Neuroinflammation. 2012. PMID: 22781194 Free PMC article.
-
Bacterial meningitis complicating the course of liver cirrhosis.Infection. 2017 Dec;45(6):795-800. doi: 10.1007/s15010-017-1039-7. Epub 2017 Jun 14. Infection. 2017. PMID: 28616745
-
Metformin mediates neuroprotection and attenuates hearing loss in experimental pneumococcal meningitis.J Neuroinflammation. 2019 Jul 27;16(1):156. doi: 10.1186/s12974-019-1549-6. J Neuroinflammation. 2019. PMID: 31351490 Free PMC article.
-
Thrombopoietin contributes to neuronal damage in experimental bacterial meningitis.Infect Immun. 2011 Feb;79(2):928-36. doi: 10.1128/IAI.00782-10. Epub 2010 Dec 13. Infect Immun. 2011. PMID: 21149592 Free PMC article.
References
-
- Bert, B., E. Dere, N. Wilhelmi, H. Kusserow, F. Theuring, J. P. Huston, and H. Fink. 2005. Transient overexpression of the 5-HT1A receptor impairs water-maze but not hole-board performance. Neurobiol. Learn. Mem. 84:57-68. - PubMed
-
- Bogdan, C. 2001. Nitric oxide and the regulation of gene expression. Trends Cell Biol. 11:66-75. - PubMed
-
- Bohr, V., O. B. Paulson, and N. Rasmussen. 1984. Pneumococcal meningitis. Late neurologic sequelae and features of prognostic impact. Arch. Neurol. 41:1045-1049. - PubMed
-
- Boje, K. M. 1995. Inhibition of nitric oxide synthase partially attenuates alterations in the blood-cerebrospinal fluid barrier during experimental meningitis in the rat. Eur. J. Pharmacol. 272:297-300. - PubMed
-
- Boje, K. M. 1996. Inhibition of nitric oxide synthase attenuates blood-brain barrier disruption during experimental meningitis. Brain Res. 720:75-83. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

